Diagnostic performance of fluorine-18 fluorodeoxyglucose positron emission tomography in the management of solitary pulmonary nodule: a meta-analysis
Introduction
A solitary pulmonary nodule (SPN) is defined as a rounded or irregular focal pulmonary opacity, measuring up to 30 mm in diameter in absence of atelectasis, obstructive pneumonia or mediastinal lymphadenopathy (1). Usually, it is found incidentally at unrelated imaging studies with a prevalence on chest X-ray of 0.09–0.2%. However, SPN are seen more often on computed tomography (CT) scans with a real overall prevalence up to 51% (2), reflecting both a technological improvement of methodology (resolution, spatial acquisition) (3) and a social change in lifestyle and habits (increased tobacco consumption, increased pollution, infectious disease relapse). Moreover, the finding of a pulmonary nodule can result in considerable repercussions and lead to unjustified alarming in case of benign incidental findings. The initial step after discovery of a pulmonary nodule is to determine its cause and characterize its nature according to features, such as size, morphology, rate of growth and by proposed risk models (4,5). CT often has led to a significant overlap between benign and malignant nodules resulting in useless invasive procedures, such as surgical biopsies (6). Integrated fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) has been widely investigated in SPNs management (7,8) and it is reported as an accurate non-invasive test (9). However, 18F-FDG-PET/CT presents a significant discordance between sensitivity (Se) and specificity (Spe) due to the presence of different conditions causing false positive (FP) or false negative (FN) results (10). Aim of this systematic review and meta-analysis is to evaluate diagnostic accuracy of 18F-FDG-PET/CT in SPN management.
Methods
Search strategy and inclusion criteria
A computerized research was carried out by three of the authors in order to investigate relevant articles, published between September 2012 and September 2017 in PubMed and Google Scholar. The MeSH research of combination of relevant keywords was as follows: [“solitary pulmonary nodule” (MeSH Terms)] OR [“solitary” (All Fields) AND “pulmonary” (All Fields) AND “nodule” (All Fields)] OR “solitary pulmonary nodule” [(All Fields) AND FDG (All Fields) AND PET (All Fields)] AND [“2012/09/24” (PDat): “2017/09/22” (PDat)]. All potential articles were checked to determine if they fulfilled the following expected inclusion criteria: (I) presence of a SPN; (II) SPNs characterization by 18F-FDG-PET/CT; (III) diagnostic SPNs characteristics clearly reported; (IV) presence of both SPNs visual and SUVmax (single and double-time evaluation) assessment as diagnostic predictors of malignancy; (V) data clearly reported to construct contingency tables in order to assess true positive (TP), FP, true negative (TN) and FN results; (VI) article published in English. Reviews, letters or case reports were excluded as much as reports in other languages. Data were extracted by two independent reviewers and included the following informations: first author, year of publication, study design (retrospective or prospective), number of enrolled patients, nodule’s biological behaviour (malignant and benign), method of diagnosis confirmation (clinical follow up or histological examination), instrumentation technology, operating protocol (radiopharmaceutical dosage and scan time), and diagnostic results expressed as TP, FP, TN and FN values.
Statistical analysis
The meta-analysis was conducted with Microsoft Excel 2016 (Microsoft®, Redmond, USA) and with IBM SPSS version 20.0 (IBM®, Segrate MI, Italy). In accordance with the extracted data, 2×2 contingency tables were derived to determine Se, Spe, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR) and accuracy index (AI). All statistics have reported as absolute values with their 95% confidence interval (95% CI). Se and Spe were calculated on the basis of the formulas TP/TP + FN and TN/TN + FP, respectively. PPV is expressed by the TP/TP+FP ratio, while NPV from the TN/TN + FN one. Likelihood ratios, as deriving both from Se and Spe, have expressed specifically by Se/(1-specificity) and (1-sensitivity)/Spe. Moreover, DOR was calculated using the TP×TN/FP×FN formula, while the AI resulted from (TP + FN)/(TP + FN + FP + TN). Se, Spe, DOR and accuracy Forest plots were constructed for both single and cumulative references. Finally, a summary ROC curve was derived to test diagnostic performance. In addition, publication bias was evaluated by a funnel plotting asymmetry test with relative standard error (SE), 95% limit, 99.7% limit and a trend line with its relative p-coefficient. A P less than 0.05 indicated the presence of asymmetry and therefore of bias.
Results
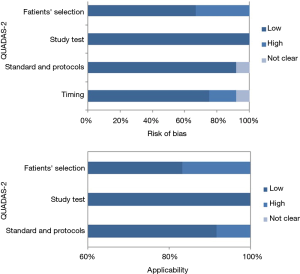
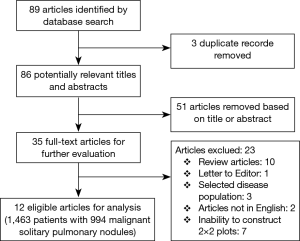
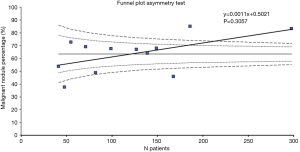
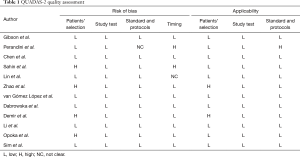
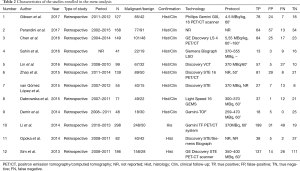
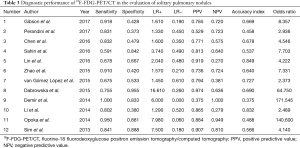
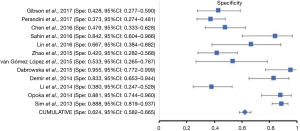
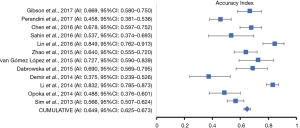
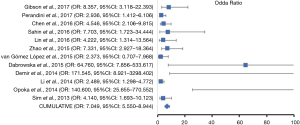
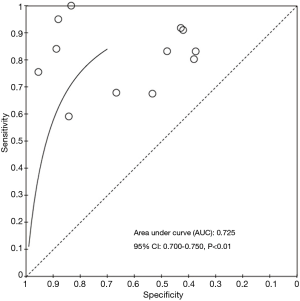
The initial research discovered 89 relevant articles, whose titles and abstracts were reviewed by two independent authors. Three articles were immediately excluded due to duplicate records. Of 68 potentially valuable articles, 51 were removed according to their titles or abstracts. Concerning with the remaining 35 papers, a second-step analysis was conducted throughout a full-text evaluation and resulting in only twelve eligible studies (11-22). In fact, 21 articles were excluded due to: (I) form incompatibility (ten review articles and one letter to editor); (II) population bias or studies conducted on selected clusters (three articles); (III) language incompatibility (two articles) and finally, (IV) inability to construct a contingency plot due to lack of data (seven articles). The quality assessment of the twelve enrolled studies was carried out according to QUADAS-2 criteria (http://www.gimbe.org/pagine/1101/it/quadas2), as reported in Table 1 sources of bias were found in patients’ selections for four articles and timing of study for two ones. Moreover, an article presented is not clear concerning with time. In regard with applicability, two studies presented at high risk; while, for standard and protocols, only one report had a high propensity issue (Figure 1). The selection progress was reported in Figure 2 and results into 1,463 patients with 994 malignant SPNs. All studies were retrospective ones and embracing a period of observation ranging from 2002 and 2015. One study lacked of this data. The definite biological behaviour of SPNs was assessed on the basis of histopathological findings or both clinical and radiological follow-up. All patients were intravenously injected with 18F-FDG and scan time was between fifty and sixty minutes. SUVmax was reported as direct visual assessment in eleven studies, while in one a double-time evaluation was accomplished. Instrumentation and operative protocols are described in detail in Table 2. Finally, overall TP results were found in 833 patients, FP in 206, FN in 183 and TN ones in 343, respectively. Concerning with 18F-FDG-PET/CT diagnostic performance in the evaluation of SPNs, the comparison between malignant and benign ones resulted that the pooled Se, Spe, PLR, NLR, PPV, NPV and AI with relative 95% confidence intervals were 0.819 (95% CI: 0.794–0.843), 0.624 (95% CI: 0.582–0.665), 2.190 (95% CI: 1.950–2.440), 0.290 (95% CI: 0.250–0.330), 0.802 (95% CI: 0.783–0.819), 0.652 (95% CI: 0.618–0.684) and 0.649 (95% CI: 0.625–0.673), respectively. The DOR was 7.049 with a relative 95% CI between 5.550 and 8.944 (Table 3). For sensibility, Spe, AI and odds ratio Forest plots were derived (Figures 3-6). Moreover, a summarized ROC curve was constructed and its relative AUC was 0.725 (95% CI: 0.700–0.750, P<0.01) (Figure 7). Finally, in regards to publication bias, derived P funnel plot was of 0.3057 confirming the absence of bias as reported in Figure 8.
Full table
Full table
Full table

Discussion
In the US, the incidence of SPNs as a result of different examinations carried out for various clinical conditions, has dramatically increased and is estimated around 1.6 million cases (23). A SPN is characterized as a single focal, predominantly peripheral, opacity <3 cm in diameter (24) and it may indicate a primitive lung cancer that represents the first worldwide cause of related-tumor death (25). Therefore, a proper management of SPNs is crucial in predicting malignancy in order to plan the most appropriate invasive and non-invasive diagnostic strategies (26). Concerning with these letters, the role of imaging and in particular of functional examinations is indispensable (7,27). 18F-FDG-PET and combined PET/CT using FDG are two widely used imaging techniques in oncology and the investigation of SPN has rapidly become one of the main indications for such imaging, although there are not negligible rates of false positives results (10–25%) as in many inflammatory or infectious diseases. In addition, some neoplasms such as carcinoid, solid adenocarcinoma, minimally invasive carcinomas, and atypical adenomatous hyperplasia (AAH) presents as false findings due to their reduced glucose metabolism (28-30). In addition, for the solitary pulmonary ground glass opacities (GGO) as reported by Song et al. (31) in a study on 72 patients, no statistically significant differences between CT and PET/CT staging were found. Thus suggested that in patients with GGO, PET/CT should be omitted in order to avoid further diagnostic procedures in the case of false positive findings. As it is known, the incidence of both false positives and negative results significantly affects the diagnostic accuracy of an examination and therefore its clinical validity. However, according to our systematic review and meta-analysis PET/CT has a good AI (AI: 0.65), with a pooled Se and Spe for malignant SPNs of 0.82 and 0.62 respectively. Moreover, the area under curve (AUC) at the summarized ROC curve was 0.729 with a statistically significant diagnostic performance (P<0.01). Cronin et al. (32), in a meta-analysis involving 2,867 patients with 2,896 nodules, reported an overall sensibility of 0.95 (0.93–0.98) and Spe of 0.82 (0.77–0.88) for PET/CT. Barger et al. (33), conducting a meta-analysis about a comparison between single and dual time PET-CT in SPN patients (816 patients with 890 SPN), showed a sensibility of 0.85 (0.82–0.89) with a lower Spe of 0.77 (0.72–0.81) due to a high heterogeneity in samples and results. Moreover, Ruilong et al. (34), in a recent systematic review involving 1,297 and 1,301 SPNs reported good but not excellent pooled Se and Spe of 0.82 (95% CI: 0.76–0.87) and 0.81 (95% CI: 0.66–0.90) respectively and suggesting 18F-FDG-PET/CT should be considered a useful diagnostic tool for malignant pulmonary nodules qualitative assessment with only a moderate accuracy in differentiating malignant from benign ones, which is consistent with our analysis. These results agree with those from Zhao et al. (35) that, in a study involving 13 published articles for a total of 962 patients, reported an overall sensibility and Spe of 0.80 (95% CI: 0.76–0.84, I2=83.2%) and 0.75 (95% CI: 0.71–0.79, I2=89.3%), respectively. These results are also confirmed at the positive and negative likelihood analysis. Concerning with the AUC at ROC, we identified a cut-off of 0.729 (95% CI: 0.700–0.750, P<0.01). Although a statistically significance was found in diagnostic performance, it appeared a slight inferior than that published by Ruilong et al. (34), who identified an AUC value of 0.87 (95%CI: 0.84−0.90). The reasons can be found in the differences from pooled Spe between studies. In fact, among the twelve enrolled articles, two of them (12,15) presented significantly inferior Spe rates than other (mean Spe 0.38) and thus affecting overall rate. According to our results, a LR+ of 2.19 (1.95–2.44) confirmed only small influence in likelihood diagnostic disease by adopting 18F-FDG-PET/CT for SPNs evaluation and this contrasts with those reported by Cronin et al. (32), who found a LR+ of 5.44 (3.56–7.32) at 18F-FDG-PET/CT. Moreover, our LR− of 0.29 (0.25–0.33) suggests a relative but still limited role in discrimination FN from TP results. Our results, however, are confident with those from Barger et al. (33), who reported a LR+ of 2.7 (CI: 1.4–5.2) and a LR− of 0.26 (CI: 0.26–0.49). For these reasons, it should be not considered as the single deciding factor to assess SPNs malignancy and in fact, its indication for SPNs management is based on prediction models of malignancy in order to avoid invasive procedures in patients with obvious benign nodules. In this regard, current guidelines encourage the adoption of Bayesian models (36,37). The American College of Chest Physician (ACCP) classes patients into 3 groups: very low likelihood (<5%), low to moderate likelihood (5–60%), and high likelihood (>60%) (4) and, according to risk assessment, surveillance is recommended for low-probability nodules, further diagnostic examinations for intermediate risk patients and invasive procedures for high risk ones (37). Models, however, should be considered as indicative and non-discriminatory. In fact, the likelihood of risk and hence the adoption of radiological and nuclear tests, as reported by the same ACCP Guidelines (38), should be correlated to the local setting (demographic variables and risk factor predominance), the risk of over-diagnosis and over-treatment and cost/effective ratios, through a critical review of the recommendations. In this regard, Asian guidelines (39) have been recently published due to the high prevalence of endemic infectious regions affecting the actual incidence of malignant pulmonary nodules. However, the high prevalence of infectious nodules (tuberculosis) also requires a definitive diagnosis both for the treatment of the patient and for reasons of public health. This revision therefore seeks to redefine radiological indications for subsequent invasive procedures in patients with inflammatory conditions. In the case of solid pulmonary nodules of greater than 8mm in size, it is recommended both to refer to a first-level center with the adoption of 18F-FDG-PET/CT in order to estimate the probability of risk and guide to subsequent invasive studies. In this pattern, radiological surveillance is recommended in low-risk patients; while, for moderate-risk ones (from 5% to 60% of risk), 18F-FDG-PET/CT should be mandatory. This cost-effectiveness strategy relies on geographical reasons (technological availability and costs), the high number of false positive and FN findings (TB, fungemia, parasitic diseases) and the high incidence of low metabolic neoplasms (adenocarcinoma). For these reasons, 18F-FDG-PET/CT does not represent a discriminative means in Asia and biopsy (surgical or radiological) remains, however, justified. On the other hand, its role in staging high-risk patients is clear (>60%). For sub-solid nodules, however, PET should be recommended but not mandatory in patients with >8 mm nodules, as an additional study for staging disease. The American College of Chest Physicians (ACCP) guidelines suggest a clinical and instrumental approach depending on the size of the pulmonary nodule (37). In individuals with solid lung nodules >8 mm and with low to moderate risk of malignancy (from 5% to 60%), 18F-FDG-PET/CT is recommended; while in patients with partially solid solitary pulmonary nodules (psSPNs) greater than 8mm, PET is recommended only after a 3-month follow-up CT. According to the British Society of Thoracic Surgery (BTS) (36), 18F-FDG-PET/CT should be contemplated in patients with SPNs >10 mm and a malignancy risk greater than 10% (Brock model). On the contrary, neither the European Association of Nuclear Medicine (EANM) nor the Society of Nuclear Medicine and Molecular Imaging (SNMMI) guidelines provide a recommendation for the execution of PET/CT in patients with SPN (40,41). Several limitations of this study should be not ignored. First, the studies included are retrospective and could be affected by selection bias. Second, the number of patients involved is relative small and therefore, the results may have been overestimated. Third, 586 patients came from three articles with low 18F-FDG-PET/CT Spe and thus reflecting also in the overall one. In particular, these studies, all from Asian countries, presented a predominance of adenocarcinomatous SPN leading to low Spe rates. Forth, confirmation strategies were heterogeneous due to the presence of both radiological follow up and histology. However, according to our funnel plot, no publication bias was found.
Conclusions
The meta-analysis shows that PET/CT has a good diagnostic accuracy in SPNs evaluation, yielding satisfactory Se and Spe. However, it should not be considered as a discriminatory test rather than a method to be included in a clinical and diagnostic pathway that cannot disregard the epidemiological and demographic aspects. Finally, a proper indication, according to stratification of risk and population bias, would increase accuracy and therefore its predictive value of malignancy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Gohagan J, Marcus P, Fagerstrom R, et al. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the Lung Screening Study of the National Cancer Institute. Chest 2004;126:114-21. [Crossref] [PubMed]
- Coenen A, Honda O, van der Jagt EJ, et al. Computer-assisted solid lung nodule 3D volumetry on CT: influence of scan mode and iterative reconstruction: a CT phantom study. Jpn J Radiol 2013;31:677-84. [Crossref] [PubMed]
- Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:108S-30S.
- Henschke CI, Yankelevitz DF, Libby DM, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. [Crossref] [PubMed]
- Bogot NR, Shaham D. Semi-invasive and invasive procedures for the diagnosis and staging of lung cancer. II. Bronchoscopic and surgical procedures. Radiol Clin North Am 2000;38:535-44. [Crossref] [PubMed]
- Divisi D, Barone M, Zaccagna G, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography in the management of solitary pulmonary nodule: a review. Ann Med 2017;49:626-35. [Crossref] [PubMed]
- Mosmann MP, Borba MA, de Macedo FP, et al. Solitary pulmonary nodule and 18F-FDG PET/CT. Part 1: epidemiology, morphological evaluation and cancer probability. Radiol Bras 2016;49:35-42. [Crossref]
- Gould MK, Maclean CC, Kuschner WG, et al. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 2001;285:914-24. [Crossref] [PubMed]
- Ambrosini V, Nicolini S, Caroli P, et al. PET/CT imaging in different types of lung cancer: an overview. Eur J Radiol 2012;81:988-1001. [Crossref] [PubMed]
- Gibson G, Ravi Kumar A, Steinke K, et al. Risk stratification in the investigation of pulmonary nodules in a high risk cohort - PET/CT outperforms clinical risk prediction algorithms. Intern Med J 2017;47:1385-92. [Crossref] [PubMed]
- Perandini S, Soardi GA, Larici AR, et al. Multicenter external validation of two malignancy risk prediction models in patients undergoing 18F-FDG-PET for solitary pulmonary nodule evaluation. Eur Radiol 2017;27:2042-6. [Crossref] [PubMed]
- Chen S, Li X, Chen M, et al. Limited diagnostic value of Dual-Time-Point (18) F-FDG PET/CT imaging for classifying solitary pulmonary nodules in granuloma-endemic regions both at visual and quantitative analyses. Eur J Radiol 2016;85:1744-9. [Crossref] [PubMed]
- Şahin E, Kara A, Elboğa U. Contribution of nonattenuation-corrected images on FDG-PET/CT in the assessment of solitary pulmonary nodules. Radiol Med 2016;121:944-9. [Crossref] [PubMed]
- Lin KH, Lee RC, Liu RS, et al. The prognostic value of tumor shadow disappearance rate on integrated PET/CT evaluation of solitary pulmonary nodules with low glucose metabolism. Nucl Med Commun 2016;37:356-62. [Crossref] [PubMed]
- Zhao M, Chang B, Wei Z, et al. The role of 18F-FDG uptake features in the differential diagnosis of solitary pulmonary lesions with PET/CT. World J Surg Oncol 2015;13:271. [Crossref] [PubMed]
- van Gómez López O, García Vicente AM, Honguero Martínez AF, et al. (18)F-FDG-PET/CT in the assessment of pulmonary solitary nodules: comparison of different analysis methods and risk variables in the prediction of malignancy. Transl Lung Cancer Res 2015;4:228-35. [PubMed]
- Dabrowska M, Krenke R, Korczynski P, et al. Diagnostic accuracy of contrast-enhanced computed tomography and positron emission tomography with 18-FDG in identifying malignant solitary pulmonary nodules. Medicine (Baltimore) 2015;94:e666. [Crossref] [PubMed]
- Demir Y, Polack BD, Karaman C, et al. The diagnostic role of dual-phase (18)F-FDG PET/CT in the characterization of solitary pulmonary nodules. Nucl Med Commun 2014;35:260-7. [Crossref] [PubMed]
- Li S, Zhao B, Wang X, et al. Overestimated value of (18)F-FDG PET/CT to diagnose pulmonary nodules: Analysis of 298 patients. Clin Radiol 2014;69:e352-7. [Crossref] [PubMed]
- Opoka L, Kunikowska J, Podgajny Z, et al. Accuracy of FDG PET/CT in the evaluation of solitary pulmonary lesions - own experience. Pneumonol Alergol Pol 2014;82:198-205. [Crossref] [PubMed]
- Sim YT, Goh YG, Dempsey MF, et al. PET-CT evaluation of solitary pulmonary nodules: correlation with maximum standardized uptake value and pathology. Lung 2013;191:625-32. [Crossref] [PubMed]
- Gould MK, Tang T, Liu IL, et al. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med 2015;192:1208-14. [Crossref] [PubMed]
- Patel VK, Naik SK, Naidich DP, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 1: radiologic characteristics and imaging modalities. Chest 2013;143:825-39. [Crossref] [PubMed]
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Álvarez Martínez CJ, Bastarrika Alemañ G, Disdier Vicente C, et al. Guideline on management of solitary pulmonary nodule. Arch Bronconeumol 2014;50:285-93. [Crossref] [PubMed]
- Truong MT, Ko JP, Rossi SE, et al. Update in the evaluation of the solitary pulmonary nodule. Radiographics 2014;34:1658-79. [Crossref] [PubMed]
- Jeong YJ, Yi CA, Lee KS. Solitary Pulmonary Nodules: Detection, Characterization and Guidance for Further Diagnostic Workup and Treatment. AJR Am J Roentgenol 2007;188:57-68. [Crossref] [PubMed]
- Nomori H, Watanabe K, Ohtsuka T, et al. Evaluation of F-18 FDG PET scanning for pulmonary nodules less than 3 cm in diameter with special reference to the CT images. Lung Cancer 2004;45:19-27. [Crossref] [PubMed]
- Erasmus JJ, McAdams HP, Patz EF, et al. Evaluation of primary pulmonary carcinoid tumors using positron emission tomography with F-18-flourodeoxyglucose. AJR Am J Roentgenol 1998;170:1369-73. [Crossref] [PubMed]
- Song JU, Song J, Lee KJ, et al. Are there any additional benefits to performing positron emission tomography/computed tomography scans and brain magnetic resonance imaging on patients with ground-glass nodules prior to surgery? Tuberc Respir Dis (Seoul) 2017;80:368-76. [Crossref] [PubMed]
- Cronin P, Dwamena BA, Kelly AM, et al. Solitary pulmonary nodules and masses: a meta-analysis of the diagnostic utility of alternative imaging tests. Eur Radiol 2008;18:1840-56. [Crossref] [PubMed]
- Barger RL Jr, Nandalur KR. Diagnostic performance of dual-time 18F-FDG PET in the diagnosis of pulmonary nodules: a meta-analysis. Acad Radiol 2012;19:153-8. [Crossref] [PubMed]
- Ruilong Z, Daohai X, Li G, et al. Diagnostic value of 18F-FDG-PET/CT for the evaluation of solitary pulmonary nodules: a systematic review and meta-analysis. Nucl Med Commun 2017;38:67-75. [Crossref] [PubMed]
- Zhao M, Ma Y, Yang B, et al. A meta-analysis to evaluate the diagnostic value of dual-time-point F-fluorodeoxyglucose positron emission tomography/computed tomography for diagnosis of pulmonary nodules. J Cancer Res Ther 2016;12:C304-8. [Crossref] [PubMed]
- Baldwin DR, Callister ME. The British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax 2015;70:794-8. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-120S.
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Bai C, Choi CM, Chu CM, et al. Evaluation of Pulmonary Nodules: Clinical Practice Consensus Guidelines for Asia. Chest 2016;150:877-93. [Crossref] [PubMed]
- Delbeke D, Coleman RE, Guiberteau MJ, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med 2006;47:885-95. [PubMed]
- Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;42:328-54. [Crossref] [PubMed]