Tumor spread through air space, the clinical implications for T factor and effects on the disease recurrence and prognosis
The findings for the tumor spread through air space (STAS) in lung adenocarcinoma were reported by Kawakami et al. (1) and have attracted attention as unique clinical characteristics associated with a micropapillary pattern and nodal metastasis (1). In 2011, the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) revised the classification of lung adenocarcinoma to include the novel findings of molecular pathology as well as the correlation with clinical outcomes (2). STAS was clearly recognized as a novel invasive morphology defined as a micropapillary clusters, solid nests, or single cells beyond the edge of the tumor into air spaces in the surrounding lung parenchyma (3). The latest WHO Classification of Tumors of The Lung, Pleura, Thymus and Heart 4th Edition in 2015 (4) basically follow the IASLC/ATS/ERS classification. STAS is often discussed in lung adenocarcinoma, especially in association with a micropapillary pattern, and is known to influence a poor outcome, even with early stage disease (5,6). STAS is recognized in approximately 30–50% of lung adenocarcinomas (5,6). Furthermore, recent publications have reported that STAS coexistence in squamous cell carcinoma was also associated with poor clinical outcomes after surgery (7,8).
Dai et al. from Shanghai Pulmonary Hospital recently reported in the Journal of Thoracic Oncology that the existence of STAS may be useful for more precisely stratifying the prognosis of resected lung adenocarcinoma (9). Tumors between 2–3 cm in size showed a worse prognosis than smaller ones, as no decreased survival was observed in tumors <2 cm in size (9). A total of 383 and 405 subjects with stage IA adenocarcinoma based on the 7th edition of TNM classification for lung cancer as proposed by the IASLC (tumor size <3 cm) were enrolled in this study as a study cohort and validation cohort, respectively. The predominant histologic subtype of adenocarcinoma was determined based on the IASLC/ATS/ERS classification (2), and the definition of STAS was the same as that used in a previous report (3). Two independent pathologists classified STAS into single-cell, micropapillary cluster, or solid nest-predominant subtypes, and the distance between the tumor margin and the furthest STAS was also measured.
Dai et al. observed STAS in 116 (30.3%) subjects in the study cohort and 127 (31.4%) subjects in the validation cohort, and STAS was more frequently observed in male patients and in coexistence with high-grade histologic adenocarcinoma, such as micropapillary or solid subtype. The maximum estimated distance between the tumor margin and the furthest STAS was 1.35 cm in the study cohort and 0.87 cm in the validation cohort. These results were compatible with the findings of a previous report by Kadota et al. that reviewed resected small (≤2 cm) adenocarcinomas (10). Those authors showed the risk of developing locoregional as well as distant recurrence in STAS-positive small lung adenocarcinomas treated with limited resection; however, the risk was hedged by performing lobectomy (10). Dai et al. complemented Kadota et al.’s report, revealing a decreased survival in STAS-positive adenocarcinomas 2–3 cm in size in a cohort in which the majority of the subjects underwent lobectomy (9). Both the recurrence-free and overall survival curves of STAS-positive adenocarcinomas 2–3 cm in size were similar to those of stage IB disease (adenocarcinoma between 3–7 cm in size) (9). Interestingly, we reported a similar phenomenon for the analysis of lung adenocarcinoma with a micropapillary pattern (11). In our analysis, the recurrence-free survival curve of adenocarcinoma with a micropapillary pattern was equivalent to that of T2 adenocarcinoma without a micropapillary pattern, and the recurrence-free survival curve of adenocarcinoma with a micropapillary pattern as well as STAS was equivalent to that of T3 adenocarcinoma without a micropapillary pattern (11).
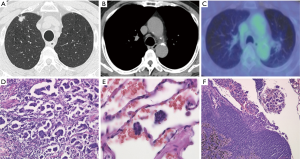
Dai et al. did not discuss the recurrence pattern in their analysis (9). Regarding recurrence patterns, it is easy to imagine the increased incidence of surgical stump recurrence in limited surgery cases due to an insufficient margin between the resection line and the STAS (12,13). The adequate resection margin has been discussed, and a distance exceeding the main tumor diameter is generally recommended (14). Therefore, achieving an adequate surgical margin is considered to be applicable for STAS-positive lung cancers, and this has also been established in previous reports (9-11). Anatomical lobectomy should be selected for STAS-positive cases, and the careful confirmation of the lack of tumor cells in the surgical stump during surgery is warranted for patients who undergo limited resection for clinical reasons. The appropriate application of techniques for examining the margin cytology might help resolve this issue (15). The positivity of nodal metastasis is another important factor influencing locoregional recurrence. Warth et al. reported the STAS was significantly associated with nodal metastasis (6). In our retrospective analysis, 15 out of 31 (48.4%) STAS-positive cases showed nodal metastasis (11). On reviewing the radiological findings of our cases, 7 out of 15 (46.7%) cases showed no nodal enlargement (<10 mm in the short axis) on chest CT as well as no abnormal accumulation of 18F-fluoro-deoxyglucose on positron emission tomography (PET) (Figure 1). Such cases with radiologically normal lymph nodes (both CT and PET negative) could be waived for any invasive nodal staging modalities, including mediastinoscopy or ultrasound-guided procedures according to the current guidelines for nodal staging in patients with lung cancer (16). The difficulty of obtaining accurate preoperative nodal staging increases the risk of not only occult N2 disease but also inappropriately selecting limited surgery.
The increased incidence of distant recurrence in STAS-positive lung cancer is another important issue. The unique and aggressive features of STAS have been shown to be associated with an increased incidence of lymphatic invasion and vascular invasion (10). A previous report showed a higher incidence of recurrence in resected stage IA non-small cell lung cancer with lymphovascular invasion than in subjects without lymphovascular invasion (17). However, the efficacy of adjuvant chemotherapy for stage IA adenocarcinoma has not been established. Indeed, 60% of patients received postoperative chemotherapy in Dai et al.’s cohort, even for the stage IA disease, but there was no significant benefit to the clinical outcome (9). Regarding the biomarkers of STAS-positive adenocarcinoma, an increased incidence of KRAS (5) and BRAF (6) mutations and a decreased incidence of epidermal growth factor receptor (EGFR) mutations (5,6,12) have been reported. This was compatible with the findings that patients with STAS are more likely to be male and smokers and to have high-grade adenocarcinoma subtypes (5,12). The genetic mechanism underlying the development of STAS by lung cancer and its relationship with aggressive invasiveness have yet to be clarified. The poor prognosis of micropapillary predominant adenocarcinoma, which is a major morphologic subtype, accompanied by STAS might be associated with mesenchymal-epithelial transition factors, as suggested by a recent publication (18). Novel treatment strategies are needed for STAS-positive patients, such as adjuvant therapy with immunocheckpoint inhibitors (19).
Diagnosing the subtypes of lung cancer is important for appropriate surgical planning; however, diagnosing STAS preoperatively or even during thoracotomy is still challenging. Attempts at detecting STAS by airway secretion cytology (20) or frozen sections during surgery (21) have been encouraging, but a number of limitations associated with high-sensitivity detection have been reported in real-world clinical settings. As mentioned above, STAS-positive lung cancer is frequently accompanied by high-grade adenocarcinoma, especially that with the micropapillary pattern (22), or squamous cell carcinoma (7,8). These tumors tend to be visualized as solid nodules on computed tomography and demonstrate a high maximum standardized uptake value on PET (23,24). When we encounter tumors with radiological features suggestive of STAS-positive lung cancer (21), we should avoid limited surgery because of the increased risk of recurrence (25).
In conclusion, Dai et al. reported that the presence of STAS was associated with a worse recurrence-free as well as overall survival in patients with stage I lung adenocarcinoma, especially for tumors 2–3 cm in size, which are currently categorized as T1c by the 8th edition of Lung Cancer TNM classification system. The survival curve was equivalent when the T factor was upgraded one level over the original one in each case. STAS is a novel concept of tumor invasion and should be routinely reported by pathologists to clinical doctors, as it drastically affects the clinical outcome. Whether or not STAS should be included in the T factor classification should be discussed in the next revision of the TNM classification system. STAS is not a rare finding and is important to consider when deciding on proper treatment strategies, especially for surgery. We need to establish better ways of categorizing patients more precisely, and Dai et al. remind us of the importance of clinical studies in lung cancer clinics.
Acknowledgements
Funding: This work was supported by JSPS KAKENHI [grant-in-aid for scientific research (C), grant number 17K10774 (T.N.)].
Footnote
Conflicts of Interest: T Nakajima received honoraria and lecture fees from Olympus Medical Systems and AstraZeneca for continuous medical education activities. The other authors have no conflicts of interest to declare.
References
- Kawakami T, Nabeshima K, Hamasaki M, et al. Small cluster invasion: a possible link between micropapillary pattern and lymph node metastasis in pT1 lung adenocarcinomas. Virchows Arch 2009;454:61-70. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Fourth edition. Lyon: International Agency for Research on Cancer, 2015.
- Onozato ML, Kovach AE, Yeap BY, et al. Tumor islands in resected early-stage lung adenocarcinomas are associated with unique clinicopathologic and molecular characteristics and worse prognosis. Am J Surg Pathol 2013;37:287-94. [Crossref] [PubMed]
- Warth A, Muley T, Kossakowski CA, et al. Prognostic Impact of Intra-alveolar Tumor Spread in Pulmonary Adenocarcinoma. Am J Surg Pathol 2015;39:793-801. [Crossref] [PubMed]
- Lu S, Tan KS, Kadota K, et al. Spread through Air Spaces (STAS) Is an Independent Predictor of Recurrence and Lung Cancer-Specific Death in Squamous Cell Carcinoma. J Thorac Oncol 2017;12:223-34. [Crossref] [PubMed]
- Kadota K, Kushida Y, Katsuki N, et al. Tumor Spread Through Air Spaces Is an Independent Predictor of Recurrence-free Survival in Patients With Resected Lung Squamous Cell Carcinoma. Am J Surg Pathol 2017;41:1077-86. [Crossref] [PubMed]
- Dai C, Xie H, Su H, et al. Tumor Spread through Air Spaces Affects the Recurrence and Overall Survival in Patients with Lung Adenocarcinoma >2 to 3 cm. J Thorac Oncol 2017;12:1052-60. [Crossref] [PubMed]
- Kadota K, Nitadori J, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Morimoto J, Nakajima T, Suzuki H, et al. Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J Thorac Cardiovasc Surg 2016;152:64-72.e1. [Crossref] [PubMed]
- Shiono S, Yanagawa N. Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I lung adenocarcinoma. Interact Cardiovasc Thorac Surg 2016;23:567-72. [Crossref] [PubMed]
- Masai K, Sakurai H, Sukeda A, et al. Prognostic Impact of Margin Distance and Tumor Spread Through Air Spaces in Limited Resection for Primary Lung Cancer. J Thorac Oncol 2017;12:1788-97. [Crossref] [PubMed]
- Sawabata N, Maeda H, Matsumura A, et al. Clinical implications of the margin cytology findings and margin/tumor size ratio in patients who underwent pulmonary excision for peripheral non-small cell lung cancer. Surg Today 2012;42:238-44. [Crossref] [PubMed]
- Utsumi T, Sawabata N, Inoue M, et al. Optimal sampling methods for margin cytology examination following lung excision. Interact Cardiovasc Thorac Surg 2010;10:434-6. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Shimada Y, Saji H, Yoshida K, et al. Pathological vascular invasion and tumor differentiation predict cancer recurrence in stage IA non-small-cell lung cancer after complete surgical resection. J Thorac Oncol 2012;7:1263-70. [Crossref] [PubMed]
- Zhang J, Sun J, Zhang Z, et al. Protein overexpression and gene amplification of cellular mesenchymal-epithelial transition factor is associated with poor prognosis in micropapillary predominant subtype pulmonary adenocarcinoma. Hum Pathol 2017. [Epub ahead of print]. [PubMed]
- Buffoni L, Vavalà T, Novello S. Adjuvant Therapy of Resected Non-small Cell Lung Cancer: can We Move Forward? Curr Treat Options Oncol 2016;17:54. [Crossref] [PubMed]
- Isaka T, Yokose T, Miyagi Y, et al. Detection of tumor spread through airspaces by airway secretion cytology from resected lung cancer specimens. Pathol Int 2017;67:487-94. [Crossref] [PubMed]
- Kameda K, Lu S, Eguchi T, et al. Can tumor spread through air spaces (STAS) in lung adenocarcinomas be predicted pre- and intraoperatively? J Thorac Oncol 2017;12:S411-2. [Crossref]
- Morales-Oyarvide V, Mino-Kenudson M. Tumor islands and spread through air spaces: Distinct patterns of invasion in lung adenocarcinoma. Pathol Int 2016;66:1-7. [Crossref] [PubMed]
- Austin JH, Garg K, Aberle D, et al. Radiologic implications of the 2011 classification of adenocarcinoma of the lung. Radiology 2013;266:62-71. [Crossref] [PubMed]
- Lee HY, Lee SW, Lee KS, et al. Role of CT and PET Imaging in Predicting Tumor Recurrence and Survival in Patients with Lung Adenocarcinoma: A Comparison with the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification of Lung Adenocarcinoma. J Thorac Oncol 2015;10:1785-94. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Indications for sublobar resection of clinical stage IA radiologic pure-solid lung adenocarcinoma. J Thorac Cardiovasc Surg 2017;154:1100-8. [Crossref] [PubMed]


