Differences in clinical presentation of non-small cell lung cancer in never-smokers versus smokers
Introduction
Among cancers, lung cancer has one of the highest incidences worldwide and non-small cell lung cancer (NSCLC) comprises a majority of it (1). Smoking has been established as a strong risk factor for lung cancer since 1950’s (2). Up to 90% of patients with lung cancer are smokers in the Western countries (3). Thus, lung cancer is often considered a smoker’s disease.
Recently, unique clinicopathologic features of NSCLC were observed in never-smokers (4,5). Numerous studies have suggested that never-smokers, compared with smokers, show a high frequency of female gender and adenocarinomas (5,6), in addition to the prolonged survival (6-8). Previous data on diverse ethnicity have also shown a high frequency of never-smokers in Asian patients compared to Western patients (9,10).
In Korea, many asymptomatic individuals are checked for their health status (11,12), and cancer screenings are frequently performed nationwide (13,14). In addition, thoracic images are widely used for benign diseases such as pulmonary tuberculosis (15,16). Thus, some patients are incidentally diagnosed with lung cancer. Of interest, despite limitations of a heterogeneous population, a previous report has suggested that incidental detection is more frequent in never-smoker than smokers (17).
In clinical practice, a precise determination on disease extent is essential for treatment modality. Timely diagnosis also remains important to prevent psychological distress (18). Although prior literature has shed insight on different clinicopathological features between smokers and never-smokers, relatively few studies have analyzed metastatic patterns and diagnostic processes based on smoking history.
Previously, a higher frequency of advanced NSCLC has been reported in never-smokers than in smokers (6,10,19). It is possible that predominance of advanced stage in never-smokers may reflect delayed diagnosis from low clinical awareness to suspect lung cancer (20). However, to our knowledge, no studies have supported this hypothesis. Insight on different tumor spreads and diagnostic processes according to smoking history may help to construct an effective strategy for further evaluation with timely diagnosis in NSCLC patients. Thus, we performed this retrospective study to evaluate different initial presentations based on smoking status.
Patients and methods
Patients and methods
We analyzed the clinical data of patients with NSCLCs who were diagnosed between January 2005 and December 2009 at two referral centers (the Korea Cancer Center Hospital in Seoul and the Hallym University Sacred Heart Hospital in Anyang). We gathered clinical data on pathologically confirmed NSCLC patients who underwent clinical staging work with brain magnetic resonance imaging (MRI), chest and upper abdominal computed tomography (CT) and additional bronchoscopy and bone scan when appropriate. Patients who had a history of malignancy within five years and those without information on smoking history excluded. However, those who received surgical resection for thyroid cancer were included. Information on smoking history was retrospectively obtained from medical records and a never-smoker was defined as someone who had never smoked in the past. Based on medical records, lung cancer with delayed diagnosis (LCDD) was defined as lung cancer without suspicion of malignancy at the initial presentation or with delayed evaluation (>4 weeks) due to a patient’s refusal. Lung cancer with transient symptom unrelated to tumors were classified as lung cancer with incidental detection (LCID), as previously described (21). Staging was determined using a new criteria (22).
Since pathological staging was not performed in all patients, T and N stages were consistently determined based on CT and additional bronchoscopic finding (23). Pleural metastases were decided using a method previously described (23). Brain MRI was used to diagnose brain metastases (24). Bone metastases were diagnosed based on multiple hot uptakes on bone scans (25) or typical findings in radiography, CT or MRI. This study was approved by the Institutional Review Board at the Korea Cancer Center Hospital and the Hallym University’s Sacred Heart Hospital.
Statistical analysis
A comparison of categorical variables based on smoking history was performed using the Mantel-Haenszel test to stratify analysis by histology (adenocarcinoma versus non-adenocarcinoma) or Pearson’s χ2 test when appropriate. Stratified analysis of continuous variables with respect to histology was also performed using Somers’ d test. Odds ratio (OR) for specific metastatic sites was obtained through logistic regression analysis adjusting for histology, T stage, and N stage. Overall survival (OS) was calculated from the time of diagnosis to the time of death from any cause. Survival curves were plotted using the Kaplan-Meier method. Log-rank tests were performed for univariate analysis. Multivariate analysis was performed using Cox regression. Stata (version 12.0; Stata Corp., College Station, TX, USA) was used for statistical analyses. The 95% confidence intervals (CIs) were determined and P values from two-sided tests <0.05 were considered significant.
Results
Patient characteristics
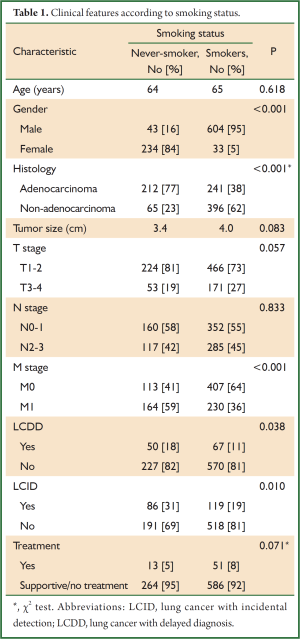
Data from 914 patients are summarized in Table 1. The median age was 65 years and 71% of the patients were men. Thirty percent of patients had never smoked. The proportions of patients with adenocarcionoma [including bronchioloalveolar carcinoma (n=14)] and squamous cell carcinoma were 50% and 37%, respectively. Primary tumors ranged in size from 0.8-15.0 cm (median, 3.7 cm). The proportions of patients with stage I, II, III, and IV were 29%, 10%, 18%, and 43%, respectively. The metastatic sites of stage IV patients included bone (n=205), contralateral lung (n=126), brain (n=118), pleura (n=108), liver (n=39), and others including adrenal gland and distant node (n=113). LCDD was identified in 117 patients (13%) who were initially diagnosed with a benign disease [pulmonary tuberculosis (n=35), pneumonia/abscess (n=43), benign solitary lung nodule (n=17), unspecified disease (n=11)], or underwent delayed evaluation (n=11). LCID comprised 22% (n=205). In the majority of patients with LCID, primary tumors were detected in chest X-rays (n=190) and the reason for the thoracic evaluations were as follows: routine screenings (n=128), evaluation for benign diseases (e.g., trauma, liver abscess; n=45), surveillance for prior cancers (n=14), and preoperative evaluation for other diseases (e.g., osteoarthritis, spinal stenosis; n=8).
Full table
Clinical features associated with smoking status
Associations between clinical features and smoking status were evaluated; the results are listed in Table 1. Based on stratified analysis with respect to histology, distant metastases (M1) were more common in never-smokers than in smokers (59% and 36%, respectively; P<0.001). Although LCDD was more frequently observed in never-smokers than in smokers (18% and 11%, respectively; P=0.038), the frequency of distant metastases in patients with LCDD was not significantly different compared with those without LCDD (49% and 42%, respectively; P=0.189). In addition, LCDD was not statistically linked to advanced T [29% vs. 24% (T1-2); P=0.134] and N stage [43% vs. 44% (N0-1); P=0.838]. Of interest, never-smokers were more likely to have LCID than smokers (31% and 19%, respectively; P=0.010). Although the proportion of T1-2 stage (P=0.057) in never-smokers was higher than in smokers, this was not statistically significant. Tumors of never-smokers, compared with smokers, showed a tendency toward small size (P=0.083). Age and N stage were not associated with smoking status.
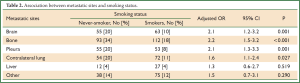
When the extent of tumor spread for metastatic sites was analyzed according to smoking status (Table 2), brain (P=0.001), bone (P<0.001), pleura (P=0.001), and lung metastases (P=0.027) were frequently detected in never-smokers compared with smokers. However, the frequency of metastases to liver and other sites were not associated with smoking history.
Full table
Survival according to smoking history
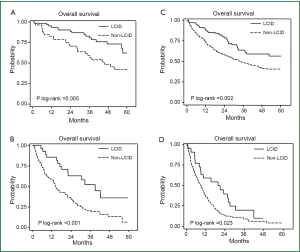
Five hundred and seventy eight deaths were observed until June 2011. Median OS of never-smokers was longer than that of smokers [30.5 months (95% CI, 24.3-36.5) and 20.2 months (95% CI, 17.4-24.2), respectively; P=0.002]. When we analyzed survival outcomes by strata using smoking history and presence of distant metastases, LCDD was not associated with survival (data not shown). In contrast, patients with LCID showed favorable outcomes within the strata (Figure 1). Similarly, in the Cox model, LCID (P=0.001; HR, 0.63; 95% CI, 0.48-0.82) remained a prognostic factor after adjusting for age, sex, smoking history, histology, and stage, whereas LCDD (P=0.987; HR, 1.00; 95% CI, 0.75-1.35) did not.
Discussion
In this study, we focused on different tumor extents and diagnostic processes according to smoking status in patients with NSCLC. In the histology-stratified analysis, never-smokers were frequently presented with distant metastases compared with smokers, which is partly consistent with previous data (6,10,19). Although never-smokers were more likely to present tumors with delayed diagnosis than smokers, this was not linked to a frequency of distant metastases at presentation. Never-smokers were also associated with a high probability of incidental detection, a favorable predictor for survival.
In the present study, never-smokers were positively associated with a frequency of distant metastases, in contrast to the advanced T and N stages. This association was significant in the analysis controlling for the effect of histology, the distribution of which is similar with other Korean studies (10,17,26). Since lung cancer is generally believed to be a smokers’ disease, a low clinical suspicion might contribute to a delayed diagnosis leading to a high frequency of advanced stage in never-smokers (20,27). Supporting this idea, we observed a high frequency of LCDD in never-smokers. This finding may indicate that physicians use inappropriate clinical thresholds to diagnose lung cancer in never-smokers. In addition, attention to pulmonary tuberculosis in a tuberculosis endemic area may be a culprit for missed diagnosis among never-smokers (15,28). However, further analysis showed no statistical association between LCDD and tumor extent. Thus, our data indicate that clinical threshold alone cannot explain the extent of tumor spread according to smoking status.
Although the incidence of LCID (22%) in this study appears high, it is within the range of previous reports (17,21,29). Considering previous data indicating a trend towards an increased number of asymptomatic patients across time periods (29), a recent series of our population may explain a high incidence of LCID. Of note, we observed a more incidental detected NSCLC in never-smokers than in smokers, which was in line with prior literature (17). This difference is significant after controlling for the effect of histology. The reason for this finding is unclear. A plausible explanation is that NSCLC in never-smokers, compared with smokers, is likely to be of the peripheral type, which is more easily detected on images than the central type (30,31). It is also possible that nicotine and smoking related nitrosamines to hyperstimulate neurotransmission may lead to biologically different tumors by releasing various molecules such as growth factors and angiogenesis factors (32-34). Further molecular studies need to be followed.
In our data, the frequency of distant metastases to the brain, pleura, bone, and lung was significantly high in never-smokers compared with smokers, whereas this finding was not observed regarding metastases to the liver and other distant organs. Although a limited number of metastases to the liver and other organs, as previously reported (35), may lead to statistical insignificance, this result indicates a potential association of smoking status with organ specificity of tumor metastases. Supporting this idea, specified metastatic patterns according to the presence of EGFR mutation, predominant in never-smokers, have been observed in NSCLC (23,36,37). Similarly, recent data have suggested links between genetic profiles and preferential metastatic sites have been suggested in other solid tumors (38-40). However, to clarify preferential metastatic sites of NSCLC in never-smokers, molecular studies are needed with further insights in biology for advanced disease.
In the survival analysis, an incidental diagnosis in both localized and advanced diseases was associated with favorable survival. The favorable survival in patients with LCID, which is partly in line with previous studies (17,21,41), may be attributable to theoretical bias such as lead-time, length-time, and over-diagnosis bias (42). Although these potential limitations are important in interpreting the benefit of screening, this is not the case for the current study. We believe that insights on different survivals according to the diagnostic process may be important. For example, the identification of incidental detection can be used for constructing therapeutic strategies and designing clinical trials for lung cancer patients. Furthermore, an increased number of incidentally detected lung cancer may be expected from wide-spread screening using thoracic examination (43,44). Thus, this study may justify a physician’s attention to the diagnostic process in NSCLC.
In addition to the retrospective nature, several other limitations can be addressed. First, we simplified delayed diagnosis based on the information at the time of referral, without consideration of the symptoms’ onset. However, a missed diagnosis apparently leading to a delay in diagnosis has been shown in a prior study (45). Indeed, there are various types of delays, and studies have used inconsistent criteria for delayed time (46). In this study, based on Canadian guidelines (47), the time from general practitioner to diagnosis was beyond the upper limit (4 weeks) in a majority of patients with LCDD (data not shown). Second, due to a limitation of CT for disease stage, particularly for the mediastinal lymph node (48), a potential bias in the distribution of N stage according to smoking status cannot be excluded. Last, a cautious interpretation is needed because the current study has been undertaken in an Asian population, who are more likely to be never-smokers than Western populations (9,10).
In conclusion, this study suggests a distinct metastatic pattern and diagnostic processes in never-smokers with NSCLC. The link between survival and incidental detection was also indicated. This finding adds new insights to understanding the clinical presentations of never-smokers. Further studies are needed to validate the results of this study.
Acknowledgements
Na II and Jang SH are the member of the Korean Thoracic Oncology Research Group (KTORG).
This work was supported in part by the Institute-Supported Research Project appointed by the Korea Cancer Center Hospital.
Disclosure: The authors declare no conflict of interest.
References
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol 2001;2:533-43. [PubMed]
- Wynder EL, Graham EA. Landmark article May 27, 1950: Tobacco Smoking as a possible etiologic factor in bronchiogenic carcinoma. A study of six hundred and eighty-four proved cases. By Ernest L. Wynder and Evarts A. Graham. JAMA 1985;253:2986-94. [PubMed]
- Parkin DM, Pisani P, Lopez AD, et al. At least one in seven cases of cancer is caused by smoking. Global estimates for 1985. Int J Cancer 1994;59:494-504. [PubMed]
- Rudin CM, Avila-Tang E, Harris CC, et al. Lung cancer in never smokers: molecular profiles and therapeutic implications Clin Cancer Res 2009;15:5646-61. [PubMed]
- Subramanian J, Velcheti V, Gao F, et al. Presentation and stage-specific outcomes of lifelong never-smokers with non-small cell lung cancer (NSCLC). J Thorac Oncol 2007;2:827-30. [PubMed]
- Toh CK, Gao F, Lim WT, et al. Never-smokers with lung cancer: epidemiologic evidence of a distinct disease entity. J Clin Oncol 2006;24:2245-51. [PubMed]
- Nordquist LT, Simon GR, Cantor A, et al. Improved survival in never-smokers vs current smokers with primary adenocarcinoma of the lung. Chest 2004;126:347-51. [PubMed]
- Tsao AS, Liu D, Lee JJ, et al. Smoking affects treatment outcome in patients with advanced nonsmall cell lung cancer. Cancer 2006;106:2428-36. [PubMed]
- Kawaguchi T, Matsumura A, Fukai S, et al. Japanese ethnicity compared with Caucasian ethnicity and never-smoking status are independent favorable prognostic factors for overall survival in non-small cell lung cancer: a collaborative epidemiologic study of the National Hospital Organization Study Group for Lung Cancer (NHSGLC) in Japan and a Southern California Regional Cancer Registry databases. J Thorac Oncol 2010;5:1001-10. [PubMed]
- Ahn MJ, Lee J, Park YH, et al. Korean ethnicity as compared with white ethnicity is an independent favorable prognostic factor for overall survival in non-small cell lung cancer before and after the oral epidermal growth factor receptor tyrosine kinase inhibitor era. J Thorac Oncol 2010;5:1185-96. [PubMed]
- Lee JW, Kang KW, Paeng JC, et al. Cancer screening using 18F-FDG PET/CT in Korean asymptomatic volunteers: a preliminary report. Ann Nucl Med 2009;23:685-91. [PubMed]
- Chong S, Lee KS, Chung MJ, et al. Lung cancer screening with low-dose helical CT in Korea: experiences at the Samsung Medical Center. J Korean Med Sci 2005;20:402-8. [PubMed]
- Yoo KY. Cancer control activities in the Republic of Korea. Jpn J Clin Oncol 2008;38:327-33. [PubMed]
- Kim Y, Jun JK, Choi KS, et al. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev 2011;12:725-30. [PubMed]
- Kim H, Kang SJ, Suh GY, et al. Predictors for benign solitary pulmonary nodule in tuberculosis-endemic area. Korean J Intern Med 2001;16:236-41. [PubMed]
- Kim HJ, Lee HJ, Kwon SY, et al. The prevalence of pulmonary parenchymal tuberculosis in patients with tuberculous pleuritis. Chest 2006;129:1253-8. [PubMed]
- In KH, Kwon YS, Oh IJ, et al. Lung cancer patients who are asymptomatic at diagnosis show favorable prognosis: a korean Lung Cancer Registry Study. Lung Cancer 2009;64:232-7. [PubMed]
- Risberg T, Sørbye SW, Norum J, et al. Diagnostic delay causes more psychological distress in female than in male cancer patients. Anticancer Res 1996;16:995-9. [PubMed]
- Ou SH, Ziogas A, Zell JA. Asian ethnicity is a favorable prognostic factor for overall survival in non-small cell lung cancer (NSCLC) and is independent of smoking status. J Thorac Oncol 2009;4:1083-93. [PubMed]
- Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer 2007;7:778-90. [PubMed]
- Raz DJ, Glidden DV, Odisho AY, et al. Clinical characteristics and survival of patients with surgically resected, incidentally detected lung cancer. J Thorac Oncol 2007;2:125-30. [PubMed]
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260-71. [PubMed]
- Na II, Park JH, Choe du H, et al. Association of epidermal growth factor receptor mutations with metastatic presentations in non-small cell lung cancer. ISRN Oncol 2011;2011:756265.
- Na II, Lee TH, Choe DH, et al. A diagnostic model to detect silent brain metastases in patients with non-small cell lung cancer. Eur J Cancer 2008;44:2411-7. [PubMed]
- Erturan S, Yaman M, Aydin G, et al. The role of whole-body bone scanning and clinical factors in detecting bone metastases in patients with non-small cell lung cancer. Chest 2005;127:449-54. [PubMed]
- Hwangbo B, Kim SK, Lee HS, et al. Application of endobronchial ultrasound-guided transbronchial needle aspiration following integrated PET/CT in mediastinal staging of potentially operable non-small cell lung cancer. Chest 2009;135:1280-7. [PubMed]
- Scagliotti GV, Longo M, Novello S. Nonsmall cell lung cancer in never smokers. Curr Opin Oncol 2009;21:99-104. [PubMed]
- Singh VK, Chandra S, Kumar S, et al. A common medical error: lung cancer misdiagnosed as sputum negative tuberculosis. Asian Pac J Cancer Prev 2009;10:335-8. [PubMed]
- Buccheri G, Ferrigno D. Lung cancer: clinical presentation and specialist referral time. Eur Respir J 2004;24:898-904. [PubMed]
- Mascaux C, Peled N, Garg K, et al. Early detection and screening of lung cancer. Expert Rev Mol Diagn 2010;10:799-815. [PubMed]
- Sone S, Takashima S, Li F, et al. Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet 1998;351:1242-5. [PubMed]
- Al-Wadei HA, Plummer HK 3rd, Ullah MF, et al. Social stress promotes and γ-aminobutyric acid inhibits tumor growth in mouse models of non-small cell lung cancer. Cancer Prev Res (Phila) 2012;5:189-96. [PubMed]
- Schuller HM. Effects of tobacco constituents and psychological stress on the beta-adrenergic regulation of non-small cell lung cancer and pancreatic cancer: implications for intervention. Cancer Biomark 2013;13:133-44. [PubMed]
- Schuller HM. Neurotransmission and cancer: implications for prevention and therapy. Anticancer Drugs 2008;19:655-71. [PubMed]
- Kagohashi K, Satoh H, Ishikawa H, et al. Liver metastasis at the time of initial diagnosis of lung cancer. Med Oncol 2003;20:25-8. [PubMed]
- Li Z, Lu J, Zhao Y, et al. The retrospective analysis of the frequency of EGFR mutations and the efficacy of gefitinib in NSCLC patients with brain metastasis. J Clin Oncol 2011;29:e18065.
- Jamal-Hanjani M, Spicer J. EGFR tyrosine kinase inhibitors in the treatment of EGFR mutant NSCLC metastatic to the brain. Clin Cancer Res 2012;18:938-44. [PubMed]
- Bos PD, Zhang XH, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature 2009;459:1005-9. [PubMed]
- Minn AJ, Gupta GP, Siegel PM, et al. Genes that mediate breast cancer metastasis to lung. Nature 2005;436:518-24. [PubMed]
- Gourley C, Michie CO, Roxburgh P, et al. Increased incidence of visceral metastases in scottish patients with BRCA1/2-defective ovarian cancer: an extension of the ovarian BRCAness phenotype. J Clin Oncol 2010;28:2505-11. [PubMed]
- Kondo R, Yoshida K, Kawakami S, et al. Different efficacy of CT screening for lung cancer according to histological type: analysis of Japanese-smoker cases detected using a low-dose CT screen. Lung Cancer 2011;74:433-40. [PubMed]
- Black WC. Overdiagnosis: An underrecognized cause of confusion and harm in cancer screening. J Natl Cancer Inst 2000;92:1280-2. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- New York Early Lung Cancer Action Project Investigators. CT Screening for lung cancer: diagnoses resulting from the New York Early Lung Cancer Action Project. Radiology 2007;243:239-49. [PubMed]
- Singh H, Hirani K, Kadiyala H, et al. Characteristics and predictors of missed opportunities in lung cancer diagnosis: an electronic health record-based study. J Clin Oncol 2010;28:3307-15. [PubMed]
- Jensen AR, Mainz J, Overgaard J. Impact of delay on diagnosis and treatment of primary lung cancer. Acta Oncol 2002;41:147-52. [PubMed]
- Simunovic M, Gagliardi A, McCready D, et al. A snapshot of waiting times for cancer surgery provided by surgeons affiliated with regional cancer centres in Ontario. CMAJ 2001;165:421-5. [PubMed]
- Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med 2003;139:879-92. [PubMed]


