Role of indacaterol, a once-daily bronchodilator, in chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is an inflammatory disease of the lung characterized by progressive airflow obstruction that is not fully reversible (1). Despite the improvements in medication and public education, COPD is still a serious health problem in the United States and around the world (2,3). In 2008, chronic lower respiratory diseases, which are primarily due to COPD, were the 3rd leading cause of mortality. It is estimated that the direct economic cost of COPD and asthma in 2008 is approximately $53.7 billion (3). Prior to 2000, studies show there was an increased rate of hospitalization and an age-adjusted mortality rate for COPD. Since 1999, there appears to have been a small decrease in age-adjusted mortality in men, but not in women. This reduction in mortality in COPD appears to correlate with the decreased prevalence in smoking, which has been steadily declining since 1965 (3). In 2010, the prevalence of smoking, the dominant risk factor for COPD, was 21.5% in men and 17.3% in women. In 2011, an estimated 6.5% of U.S. adults (approximately 13.7 million) were diagnosed with COPD. Between 1999-2011, the prevalence appeared to decline. Since much of this data is based on self-reporting, the true prevalence of COPD, based on spirometry, and the actual mortality rate are likely underestimated (3).
Lung damage from COPD has several causes including proinflammatory mediators, oxidative stress, and proteolytic digestion of the lung tissue (1). The repetitive damage of the lungs leads to a slow and gradual progression of obstruction to airflow. Although initially asymptomatic, the continued destruction of airway and lung parenchyma with subsequent worsening airflow obstruction leads to the development of progressive symptoms of cough, dyspnea, wheezing and chest tightness (3). While there is clinical variability, most patients have a progression of disease severity leading to an acceleration in sensation of dyspnea, decrease in physical activity and social functioning which correlates with a decline in forced expiratory volume in 1s (FEV1) (3). Acute exacerbations of COPD can cause a rapid decline in lung function and increased dyspnea leading to poor quality of life, increased hospitalization and mortality (4). A number of pharmacologic interventions have been developed to improve lung function, as well as, decrease dyspnea and exacerbation rates (5). Long and short acting beta2-agonists (β2-agonists), long and short acting anticholinergics, inhaled corticosteroids and phosphodiesterase inhibitors are now the cornerstones of treatment for COPD.
Current guidelines
Once COPD is diagnosed, pharmacologic and non-pharmacologic interventions are recommended depending on symptoms and disease severity. The goals of pharmacologic therapy are to improve lung function, and quality of life while reducing daily symptoms, and exacerbation rates. The current Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommends the use of long-acting bronchodilators as first-line maintenance treatment for moderate COPD and beyond (6).
The prolonged duration of action of long-acting bronchodilators promotes medication adherence and compliance thus leading to improved clinical outcomes in COPD patients (5). Studies have shown that there is a strong correlation with medication adherence and dosing frequency (7). Currently available long-acting bronchodilators in the US include: twice-daily long-acting β-agonists (LABA), formoterol, arformoterol, and salmeterol; once-daily long acting anticholinergic (LAMA), tiotropium; twice daily LAMA, aclidinium, and indacaterol (5). Indacaterol is a once-daily LABA that was developed for maintenance therapy in patients with moderate to severe COPD. The European Union has approved indacaterol at a recommended dose of 150 μg once daily with a maximal dose of 300 μg once daily. In the United States, indacaterol is licensed to be used at a dose of 75 μg once daily (5).
Pharmacology
In the early twentieth century, epinephrine was introduced as a treatment of acute asthma. Modification of early catecholamine structures allowed for improved selectivity for the β2-adrenoreceptor, leading to the development of albuterol, a short acting β2-agonist (SABA). Eventually, there was development of longer-acting agents such as salmeterol and formoterol and finally the once-daily LABA, indacaterol. β2-agonists exert their effects via their ability to relax airway smooth muscle. They work by binding to active sites of β2-adrenoreceptors which are densely located on smooth muscle, resulting in the activation of adenylyl cyclase and the generation of intracellular cAMP (8). The increase in intracellular cAMP activates effector molecules such as cAMP-dependent protein kinase A which are involved in the regulation of airway smooth muscle tone (8).
Beta-agonists share a number of effects with epinephrine including: inhibiting airway smooth muscles; increasing heart rate, contraction and conduction; inhibiting mast cell degranulation; increasing glucose from glycogenolysis; increasing insulin and glucagon release, as well as, increasing or decreasing acetylcholine release (8).
β2-agonists are usually administered via inhalation, allowing for a direct route of drug delivery to the site of action at the lowest possible dose with the least systemic side effects. Studies show that the local drug concentration is the primary cause for β2-agonist’s therapeutic effects, since peak plasma concentration only accounts for a small fraction of the decrease in airway resistance (8).
Pharmacokinetic reports show that indacaterol is rapidly absorbed via the pulmonary as well as the intestinal system, since a portion of the drug is always swallowed through the oropharynx into the gastrointestinal tract (8). In vitro studies show indacaterol has a median time to reach peak serum concentration of approximately 15 minutes after single or multiple doses. Another single-dose study of indacaterol at 600 and 2,000 μg confirmed a rapid absorption with maximal serum concentration reached within 15 minutes (5).
In vitro studies show that indacaterol has a high agonist efficacy at the human β2-adrenoreceptor with a binding affinity similar to formoterol. Indacaterol has a functional selectivity to β2-adrenoreceptor over β1-adrenoreceptor that is similar to formoterol and over the β3-adrenoreceptors, which is similar to formoterol and albuterol (8). It is still unclear how LABAs are able to sustain a long bronchodilator effect. The previous thought of slow receptor dissociation does not appear to be the key to LABA’s duration of action. It appears that the faster onset of action and longer duration of action of indacaterol is likely due to its lipid membrane interactions and ability for drug partitioning into lipophilic compartments upon inhalation (5,8). Another factor that may contribute to indacaterol’s long duration of action is its high affinity for small lipid raft microdomains, which is the location where β2-adrenoreceptors are in close contact with effector molecules. It appears that indacaterol has a twofold higher affinity for these lipid rafts than salmeterol, which may contribute to its longer duration of action (8).
Toxicology evaluation of in vitro indacaterol shows no evidence of potential carcinogenicity and teratogenicity in embryo-fetal development. Multiple-dose studies of indacaterol at 400 and 800 μg for 14 days showed a rapid absorption and a mean elimination half-life >30 hrs (5).
Physiologic effects of indacaterol
Most COPD patients, especially those who continue to smoke, are known to lose lung function at an accelerated rate when compared with normal patients. Due to its ease of use and reproducibility, FEV1 is frequently used to assess this decline in lung function in order to monitor and direct appropriate treatment in COPD patients. Mean trough FEV1 has been shown to be proportional to change in health status of COPD patients (9). In one randomized placebo-controlled study over a 52-week period, all 366 patients on once-daily indacaterol at doses of 150 or 300 μg had a significant increase in FEV1 of greater than or equal to 170 mL (10). This was found to be statistically significant when compared with placebo. Another double-blind placebo controlled study of 90 patients who were treated with 300 μg of indacaterol once-daily for three weeks showed an increased FEV1 by 250 mL, which was statistically significant when compared with placebo (11). FEV1 measurements are helpful in determining treatment efficacy, however it does not reflect the full burden of COPD subjects (12).
Although FEV1 is an essential measure used in diagnosing, staging, and treatment of COPD, there have been studies showing that changes in FEV1 only partially correlate with changes in dyspnea. The mechanism of dyspnea in COPD is complex and multifactorial requiring monitoring of several factors including FEV1, functional vital capacity (FVC), and both static and dynamic lung hyperinflation to fully assess the benefit to COPD patients (13,14). Physiologic parameters such as FEV1, inspiratory capacity (IC) and total lung capacity (TLC) are used to diagnose and monitor treatment response in COPD patients (9). Static lung volumes such as IC have been purported to correlate better with exercise tolerance and dyspnea than FEV1 and FVC, as a measure of lung hyperinflation (11). A reduced ratio of IC and TLC has been shown to correlate with mortality predictions (15). This makes measurement of IC as a marker for severity and treatment response to COPD useful in treatment studies. In a study by O’Donnell, patients receiving 300 μg of indacaterol had an increase in IC of 170 mL at rest and an increase in end-exercise IC of 280 mL over placebo after three weeks of treatment (11). In another small randomized study in which 300 μg of indacaterol was used for three months as an add-on therapy, or replacement for other LABA, IC increased by 240 mL in the indacaterol treatment group. This study also showed a significant increase in six minute walk distance (6MWD), as well as, health related quality of life (HRQL) based on St. George’s Respiratory Questionnaire (SGRQ) (16).
SGRQ is a well validated, disease-specific assessment developed to evaluate the health status of patients with asthma or COPD, and is frequently used in clinical trials as an endpoint to assess treatment effect in COPD (17). Health status and dyspnea, judged by measures such as TDI and SGRQ, were noted to be improved in a 52-week study by Dahl using 300 or 600 μg of indacaterol (16). Finally a meta-analysis on indacaterol with TDI data showed treatment doses of 150, 300 and 600 μg had significantly improvement in breathlessness in COPD patients when compared to placebo (18).
Safety
A safety study performed from data on multiple trials of indacaterol with once-daily dosing of 75, 150, 300, and 600 μg, elucidated five common adverse events. These include COPD worsening, nasopharyngitis, headache, cough, and upper respiratory tract infection (19). In this study, COPD worsening was found to be the most common adverse event, however its incidence was found to be less common on all doses of indacaterol when compared with placebo. The other most common adverse events showed no consistent increase or decrease in incidence with indacaterol at all doses when compared with placebo (19).
Many different studies on indacaterol have mentioned coughing immediately following administration. This adverse effect was highlighted in a study by Donohue et al. where approximately 15-20% of patients had a cough starting within 15 seconds of drug administration and lasting less than 15 seconds, with a median duration of less than or equal to 6 seconds (19). Despite the frequency of immediate cough with medication, it was not associated with loss of efficacy, increased bronchospasm or discontinuation rates (20).
Cardiac side effects including tachycardia, QT prolongation, and palpitation have been frequently cited as a class-effect with beta-agonists. Other adverse reactions that are recognized consequences to beta2-adrenoreceptor stimulation include hypokalemia, and hyperglycemia, which can be detrimental to the heart. A RCT of 388 subjects on dosing of indacaterol up to 600 μg per day showed no clinically relevant effect on the QT interval (21). In a 52-week RCT by Chapman et al. with once-daily dosing of indacaterol, there was no effect on serum potassium level and only a slight increase in elevated blood glucose levels in the treatment group (10). Additionally an analysis of fvie RCTs showed that symptoms of anxiety, palpitations, and tachycardia were not increased with 150 and 300 μg dosing of indacaterol when compared with placebo (19). These findings show that indacaterol is well-tolerated at multiple doses with a good overall safety profile.
Jiang et al. showed that there was a dose response to adverse effects with indacaterol use. This was demonstrated with the development of more adverse events compared to placebo in a 52-week extended study of indacaterol 600 μg once-daily with risk ratio of 1.15. However it is also noteworthy that when compared with standard treatment of formoterol, a 52-week study found no more adverse events between the 600 μg of indacaterol and twice daily formoterol. Therefore, it seems that despite the adverse effects, a higher dose of indacaterol may provide better tolerance, improved patient compliance over the long term compared to formoterol (18).
Comparison studies
Several studies have been published to evaluate the bronchodilator effects and safety of indacaterol compared to currently available drugs.
Indacaterol vs. tiotropium
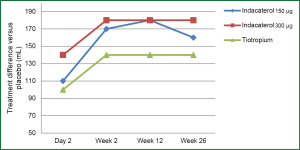
In 2010, Donohue et al. designed a randomized-controlled placebo study of 1,683 patients over a 26-week period to compare the efficacy of tiotropium and indacaterol. This was a two stage design, with initial two week stage used to determine the most efficacious dose of indacaterol by an independent committee. The 150 and 300 μg indacaterol doses were selected and compared with placebo and 18 μg of tiotropium. Spirometry was performed at baseline and at each visit. Dyspnea was evaluated based on the transition dyspnea index and health status was assessed with the SGRQ. At 12 weeks, there was an FEV1 difference versus placebo of 180 mL for both dose of indacaterol and 140 mL for tiotropium. At 26 weeks the difference in FEV1 over placebo was increased to 210 mL for 150 μg of indacaterol, 240 mL for 300 μg of indacaterol and 240 mL for tiotropium (Figure 1). Overall, indacaterol was well tolerated with headache being the most commonly reported adverse event which were generally mild to moderate. Additional adverse events include: tachycardia, which was noted in the lower dose of indacaterol; tremors, which were rarely reported, and prolonged QTc interval which were few in number and did not lead to any adverse events. Previous studies have shown a low arrhythmogenic potential for indacaterol. The study showed an improvement in dyspnea but not in health status based on mean SGRQ scores. Indacaterol maintained its bronchodilator effects over a 24-hour period of time. In this study it was felt that indacaterol was statistically noninferior to tiotropium (20). Other studies have confirmed that indacaterol is at least as effective as tiotropium and was able to show a statistically significant improvement in dyspnea and health status (based on SGRQ) with indacaterol compared to tiotropium (22).
Indacaterol vs. salmeterol
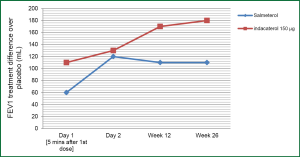
In 2011 Kornmann et al., published a study comparing the efficacy and safety of indacaterol with salmeterol. This was a placebo-controlled study where patients were randomized to 150 μg of indacaterol once-daily, 50 μg of salmeterol twice daily or placebo over a 26-week period. A total of 838 patients completed the study with a significant difference compared to placebo noted in both indacaterol and salmeterol groups. The study showed a significant change in FEV1 over placebo of 170 mL at 12 weeks and 180 mL at 26 weeks in the indacaterol group and 110 mL at 12 and 26 weeks in the salmeterol group (Figure 2). There appeared to a significant improvement in health status (based on SGRQ) at 12 weeks and improvement in dyspnea (based on TDI scores) at 4, 8, 12 and 26 weeks for indacaterol and 12 and 26 weeks for salmeterol. An evaluation done at 5 minutes after the first dose on the first day, showed an increase in FEV1 over placebo by 110 mL with indacaterol and by 60 mL with salmeterol. A similar improvement of 60-100 mL in FEV1 was observed throughout the study for indacaterol over salmeterol at 5 minutes. Adverse events noted in the study were similar across groups, with increased incidence of bacterial and viral upper respiratory tract infections in the indacaterol group (Table 1). Cough was reported on average 17.6% following inhalation of indacaterol which appears to occur within 15 seconds of inhalation and lasted approx 12 seconds. This adverse effect did not appear to be associated with increase bronchospasm or loss of efficacy (23). This study shows that once-daily indacaterol is generally superior to twice-daily salmeterol and formoterol based on trough FEV1, improvement in health status, as well as, dyspnea (23,24).
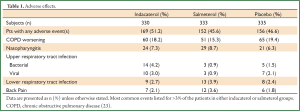
Full table
Several studies have shown that indacaterol 150 and 300 μg dosing appeared to have similar lasting effects on trough FEV1. The higher dose of indacaterol, 300 μg, appears to have an incremental benefit in improvement in dyspnea (based on transition dyspnea index) over tiotropium, both twice-daily bronchodilators, as well as, patient with more severe COPD. The safety profile and tolerability of the different dosages appear to be similar (5). Patient using indacaterol had decrease use for as-needed SABA even when compared with tiotropium or formoterol. Although not statistically significant there was a reduction in the number exacerbation compared to placebo with use of all bronchodilators (5).
In 2011, Mahler et al. compared the efficacy of tiotropium monotherapy versus indacaterol 150 μg plus tiotropium in two identical double-blind randomized controlled studies over a 12 week period. Approx 1,100 patients with moderate to severe COPD were randomized in both studies. There was a statistically significant improvement in trough FEV1 of 80 and 70 mL with the combined treatment compared to monotherapy in each study respectively. This improvement was maintained in all subgroup analyses according to COPD severity, smoking status and inhaled corticosteroid use. Although the trough FEV1 was not as large as previous studies that compared indacaterol to tiotropium, there was an incremental improvement in FEV1 and IC which shows that there is additional bronchodilator response with reduction in lung hyperinflation from combining these medications (25). The proposed mechanism of additive effects of bronchodilators includes: relaxation of smooth muscle secondary to independent effects on sympathetic and parasympathetic pathways, differential distribution of β2-adrenergic and muscarinic receptors, or the interaction of the two receptors causing a potentiation of β2-receptor activation by muscarinic receptor blockage (25).
A review article published in 2012 by Rodrigo et al. compared indacaterol (150-300 μg) with tiotropium or twice-daily LABAs. A total of five randomized controlled trials were reviewed which included approximately 6,000 participants. Two studies compared indacaterol with tiotropium and three studies compared indacaterol with twice-daily LABAs. Analysis revealed that there was no statistically significant improvement in trough FEV1 between indacaterol and tiotropium but there was a significant improvement of 80 mL in trough FEV1 with indacaterol and twice-daily LABAs. Compared with tiotropium and twice-daily LABA, indacaterol had a significant reduction in rescue albuterol use and sensation of dyspnea, which provides insight into the effectiveness of treatment. Compared with twice-daily LABA, indacaterol also had a significant improvement in health status, based on SGRQ. This study was able to quantify a number needed to treat with indacaterol of 10 patients in order to experience these clinical improvements over tiotropium or twice-daily LABAs. Although indacaterol showed improvement in health status, dyspnea and pulmonary function compared to tiotropium or twice-daily LABA, there was no significant difference in the rate of COPD exacerbation, withdrawal, adverse events or all-cause mortality (26).
Indacaterol 75 µg dose
There has been some controversy regarding the most efficacious dose for the treatment of COPD. An analysis of 801 patients with moderate to severe COPD who were treated for 2 weeks showed that 150 μg of indacaterol was the lowest dose that was superior to the active comparators, formoterol and tiotropium (27). Despite these studies, the Food and Drug Administration (FDA) approved indacaterol at a dose of 75 μg once daily. According to the FDA’s analysis of the original dose-exploration data, all doses on indacaterol fulfilled the criteria of trough FEV1 greater than 0.12 liters over placebo, as well as, produced a higher trough FEV1 with an area under the FEV1 curve 1 to 4 hours after a dose compared with other bronchodilators (28). It was felt that all doses were more effective than placebo without a significant increase in dose-response above the 75 μg dose. A 12 week placebo controlled study was performed by Novartis to compare the 75 and 150 μg doses of indacaterol; both dosages resulted in a significantly higher trough FEV1 than placebo. The study also showed a pooled analysis for HRQL (based on SGRQ) at week 12 showing a statistically significant improvement with 75 μg dose of indacaterol over placebo, but higher doses did not show any incremental benefits in quality of life. An analysis of 23 trials with over 11,755 COPD patients did not show any significant worrisome findings for indacaterol at the 75 and 150 μg doses, but there was some concern for increase asthma exacerbation and respiratory related deaths with the 300 μg of indacaterol. Due to this risk-benefit evaluation, the FDA approved the use of 75 μg dose of indacaterol in the United States (27).
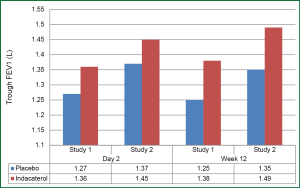
Kerwin et al., published two identical studies in 2011 comparing the efficacy of indacaterol 75 μg with placebo in a double-blind placebo-controlled study over a 12-week period. Each study evaluated approximately 320 patients and they both showed a significant improvement in trough FEV1 in the indacaterol group over placebo. The increase in trough FEV1 over placebo at 12 weeks was 120 and 140 mL for each study respectively (Figure 3). The 75 μg indacaterol dose also confirmed a rapid onset with prolong duration of action; the 5 minutes post-dose on day one was approximately 90-100 mL which had sustained efficacy throughout the day (Figure 4). Approximately half of the patients in both studies reported adverse events. The most common event in each group, which were similar to previous studies, included COPD worsening, an acute cough, headache, nasopharyngitis and rarely tachycardia or muscle spasm. Although there were two deaths in the placebo group, this was not felt to be related to the study medication. This dose of indacaterol was also able to show a significant reduction in use of rescue albuterol showing its effectiveness to control symptoms (29).
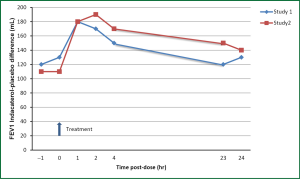
A follow-up study by Gotfried et al., was published in 2012 showing the secondary efficacy endpoints from the two double-blind, placebo-controlled 12 weeks studies published by the same authors. Both studies showed a statistically significant improvement in dyspnea, measured by TDI, at 4 weeks but only in one study at 12 weeks. Health status, measured by the SGRQ, showed a clinically relevant improvement with indacaterol from baseline across all domains. Both studies showed numerical improvement in various symptom-based end-points, a few of which were statistically significant including percentage of days able to perform usual activities, percentage of days with no daytime symptoms, percentage of nights with no awakenings, as well as, overall number of COPD exacerbations. The use of rescue albuterol use was statistically significant with indacaterol in both studies. Although not specifically powered for these end-points, these studies show a clinically relevant improvement in health status, based on SGRQ scores, compared to baseline (30).
In 2012, Cope et al. published a meta-analysis comparing indacaterol 75 μg with fixed-dose combinations of formoterol-budesonide or salmeterol-fluticasone. Based on the studies, indacaterol 75 μg resulted in a greater improvement in trough FEV1 at 12 weeks compared to formoterol-budesonide at doses of 9/160 and 9/320 μg twice-daily. This meta-analysis also showed that indacaterol 75 μg had a comparable change in trough FEV1 from baseline compared with salmeterol-fluticasone at 50/250 and 50/500 μg twice-daily doses. They concluded that indacaterol at 75 μg was at least as efficacious as formoterol-budesonide at both dosages and is comparable with salmeterol-fluticasone at both dosages (31).
In 2012, Cope et al. published another meta-analysis comparing 75 μg indacaterol with usual doses of tiotropium, salmeterol, formoterol, and placebo. Overall, there were 22 randomized controlled trials that were included in the analysis. The results of the meta-analysis showed that 75 μg of indacaterol provides an FEV1 result that was comparable to tiotropium and salmeterol at 12 weeks and a higher FEV1 versus formoterol. With regards to assessment of HRQL, based on SGRQ, all treatments were more efficacious than placebo at 12 weeks. This shows that there is a comparable level of improvement in HRQL with indacaterol 75 μg compared to tiotropium, salmeterol and formoterol (32).
Cost-effectiveness
Due to the large number of variables related to medication costs, including differing health policies, health insurance costs, medication compliance rates, pharmaceutical/commercial aspects and patient preferences, determining the cost-effectiveness of a medication is extremely difficult. Current data appears to indicate there is a difference in cost-effectiveness between bronchodilators. A recent cost-effectiveness analysis from Germany of indacaterol showed that indacaterol 150 μg is better (lower total cost with better outcomes) than tiotropium bromide or salmeterol. Another analysis comparing indacaterol 300 μg against tiotropium showed an incremental cost-effectiveness ratio of approximately 28,300 euros per quality-adjusted life years (33).
Conclusions
Indacaterol is a once-daily long-acting β2-agonist, approved for the treatment of moderate to severe COPD. It is currently approved in the United States at a dose of 75 μg and in the European Union at a recommended dose of 150 μg as well as 300 μg for severe COPD. Studies show that it has a rapid onset of action, within 5 minutes, and provides prolonged bronchodilator effects, at least 24 hours. Current studies show that indacaterol is as effective as tiotropium and superior to twice-daily LABA, salmeterol and formoterol. Higher dosing of indacaterol did provide some incremental benefit associated with improvement in trough FEV1, and dyspnea, however some studies showed a trend towards increased adverse effects. The improvement in lung measurements correlate to clinical outcomes, including improvement in dyspnea, HRQL, and exacerbation rate when compared to some of the other available bronchodilators, but mainly when compared to placebo. Several studies show that indacaterol is generally well tolerated and has a favorable safety profile when compared to other bronchodilators without significant safety issues. The most prevalent side effect is a cough which is seen within seconds of inhalation with rapid resolution. This adverse effect does not appear to cause bronchospasm or loss of efficacy. Other relevant adverse effects include: worsening COPD, upper respiratory infection, and possible increase in asthma exacerbation, and asthma-related deaths, particularly at higher doses of indacaterol. There were no significant or clinical relevant cardiac effects, including QTc prolongation, tachycardia, high blood pressure, hypokalemia or hyperglycemia. Since current guidelines recommend at least one long-acting bronchodilator as first-line maintenance therapy for moderate symptomatic COPD patients, it seems that both indacaterol, a once-daily LABA, or tiotropium, a once-daily LAMA, would be an appropriate starting medication. The once-daily dosing will provide long acting bronchodilation and promote medication compliance in order to improve patient outcomes. If symptoms are not controlled with a once-daily bronchodilator, GOLD guidelines recommend combining a LABA and LAMA. The use of indacaterol as add-on therapy has been shown to provide additional clinical improvement which will allow for a convenient treatment regimen compared to what most COPD patients currently use. This review shows that indacaterol is appropriate first-line or additive treatment option for the management of moderate to severe COPD (GOLD stage II-III) and, although not specifically studied, will likely be beneficial for late stage COPD (GOLD stage IV).
Acknowledgements
Dr. Gotfried has received research grants from Novartis, GlaxoSmithKline, and Boehringer Ingelheim and is on the Speaker Bureau for GlaxoSmithKline, Merck, Novartis, and Mylan Pharmaceuticals.
Disclosure: The authors declare no conflict of interest.
References
- Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J 2008;31:1334-56. [PubMed]
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765-73. [PubMed]
- Ford ES, Croft JB, Mannino DM, et al. COPD surveillance--United States, 1999-2011. Chest 2013;144:284-305. [PubMed]
- Yohannes AM, Connolly MJ, Hanania NA. Ten years of tiotropium: clinical impact and patient perspectives. Int J Chron Obstruct Pulmon Dis 2013;8:117-25. [PubMed]
- Ribeiro M, Chapman KR. Comparative efficacy of indacaterol in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2012;7:145-52. [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. Available online: http://www.goldcopd.org/Guidelines/guidelines-resources.html
- Toy EL, Beaulieu NU, McHale JM, et al. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med 2011;105:435-41. [PubMed]
- Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med 2013;187:690-6. [PubMed]
- Westwood M, Bourbeau J, Jones PW, et al. Relationship between FEV1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic review. Respir Res 2011;12:40. [PubMed]
- Chapman KR, Rennard SI, Dogra A, et al. Long-term safety and efficacy of indacaterol, a long-acting β2-agonist, in subjects with COPD: a randomized, placebo-controlled study. Chest 2011;140:68-75. [PubMed]
- O’Donnell DE, Casaburi R, Vincken W, et al. Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respir Med 2011;105:1030-6. [PubMed]
- Glaab T, Vogelmeier C, Buhl R. Outcome measures in chronic obstructive pulmonary disease (COPD): strengths and limitations. Respir Res 2010;11:79. [PubMed]
- Mahler DA, Weinberg DH, Wells CK, et al. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 1984;85:751-8. [PubMed]
- Taube C, Lehnigk B, Paasch K, et al. Factor analysis of changes in dyspnea and lung function parameters after bronchodilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:216-20. [PubMed]
- Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;171:591-7. [PubMed]
- Mroz RM, Minarowski L, Chyczewska E. Indacaterol add-on therapy improves lung function, exercise capacity and life quality of COPD patients. Adv Exp Med Biol 2013;756:23-8. [PubMed]
- Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis 1992;145:1321-7. [PubMed]
- Jiang FM, Liang ZA, Zheng QL, et al. Safety and efficacy of 12-week or longer indacaterol treatment in moderate-to-severe COPD patients: a systematic review. Lung 2013;191:135-46. [PubMed]
- Donohue JF, Singh D, Kornmann O, et al. Safety of indacaterol in the treatment of patients with COPD. Int J Chron Obstruct Pulmon Dis 2011;6:477-92. [PubMed]
- Donohue JF, Fogarty C, Lötvall J, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med 2010;182:155-62. [PubMed]
- Khindri S, Sabo R, Harris S, et al. Cardiac safety of indacaterol in healthy subjects: a randomized, multidose, placebo- and positive-controlled, parallel-group thorough QT study. BMC Pulm Med 2011;11:31. [PubMed]
- Buhl R, Dunn LJ, Disdier C, et al. Blinded 12-week comparison of once-daily indacaterol and tiotropium in COPD. Eur Respir J 2011;38:797-803. [PubMed]
- Kornmann O, Dahl R, Centanni S, et al. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J 2011;37:273-9. [PubMed]
- Dahl R, Chung KF, Buhl R, et al. Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax 2010;65:473-9. [PubMed]
- Mahler DA, D’Urzo A, Bateman ED, et al. Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double-blind comparison. Thorax 2012;67:781-8. [PubMed]
- Rodrigo GJ, Neffen H. Comparison of indacaterol with tiotropium or twice-daily long-acting β -agonists for stable COPD: a systematic review. Chest 2012;142:1104-10. [PubMed]
- Barnes PJ, Pocock SJ, Magnussen H, et al. Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. Pulm Pharmacol Ther 2010;23:165-71. [PubMed]
- Chowdhury BA, Seymour SM, Michele TM, et al. The risks and benefits of indacaterol--the FDA’s review. N Engl J Med 2011;365:2247-9. [PubMed]
- Kerwin EM, Gotfried MH, Lawrence D, et al. Efficacy and tolerability of indacaterol 75 μg once daily in patients aged ≥40 years with chronic obstructive pulmonary disease: results from 2 double-blind, placebo-controlled 12-week studies. Clin Ther 2011;33:1974-84. [PubMed]
- Gotfried MH, Kerwin EM, Lawrence D, et al. Efficacy of indacaterol 75 μg once-daily on dyspnea and health status: results of two double-blind, placebo-controlled 12-week studies. COPD 2012;9:629-36. [PubMed]
- Cope S, Kraemer M, Zhang J, et al. Efficacy of indacaterol 75 μg versus fixed-dose combinations of formoterol-budesonide or salmeterol-fluticasone for COPD: a network meta-analysis. Int J Chron Obstruct Pulmon Dis 2012;7:415-20. [PubMed]
- Cope S, Zhang J, Williams J, et al. Efficacy of once-daily indacaterol 75 μg relative to alternative bronchodilators in COPD: a study level and a patient level network meta-analysis. BMC Pulm Med 2012;12:29. [PubMed]
- Rutten-van Mölken MP, Goossens LM. Cost effectiveness of pharmacological maintenance treatment for chronic obstructive pulmonary disease: a review of the evidence and methodological issues. Pharmacoeconomics 2012;30:271-302. [PubMed]


