Prognostic significance of CT-determined emphysema in patients with small cell lung cancer
Introduction
Lung cancer is by far the major cause of cancer-related death worldwide and small cell lung cancer (SCLC) accounts for 15% of all cases (1), and although SCLC is highly responsive to initial chemotherapy and radiotherapy, its prognosis is poor. Chronic obstructive pulmonary disease (COPD), as a common respiratory disease, is also associated with high morbidity and mortality worldwide (2). Recent studies suggest the genetic risk factors that predispose smokers to COPD may overlap with those that predispose smokers to lung cancer (3). Emphysema is one of the classic subtypes of COPD, and is characterized by abnormal and permanent enlargement of airspaces distal to terminal bronchioles, and thus, can be visualized and quantified by computed tomography (CT) (4). Recent studies have revealed the importance of CT-diagnosed emphysema in lung cancer patients; the presence of CT diagnosed emphysema is known to be associated with increased risk of lung cancer, independent of smoking history and airflow obstruction (5). However, the relationship between emphysema and lung cancer seems to depend on histologic types. In one study, increased odds between two types of lung cancer (squamous cell carcinoma and SCLC) and emphysema was found, even though the association disappeared in SCLC after adjusting for age, sex, COPD, and smoking history (6). Furthermore, several studies reported that emphysema detected by CT negatively affects prognosis in patients with resected or combined stage non-SCLC (7-9).
To the best of our knowledge, no previously study has evaluated the prognostic significance of pulmonary emphysema in SCLC patients. Since SCLC is also prevalent in patients with a smoking history and emphysema is frequently detected in the patients (3,6), we hypothesized the severity of emphysema affects prognosis in SCLC. In the present study, we investigated the prognostic value of emphysema score as determined by baseline chest CT in SCLC patients.
Methods
Study subjects
This retrospective study was performed on 149 consecutive patients with newly diagnosed, histologically confirmed SCLC at Gachon University Gil Medical Center (Incheon, Korea) from January 2010 to December 2014. The medical records of all enrolled patients were reviewed. Patient information included age, gender, performance status (PS), smoking history, stage, treatment, chemotherapy regimen, serum lactate dehydrogenase (LDH), and spirometric and survival data. SCLC was classified as limited or extensive. The former was defined as American Joint Committee on Cancer stages I to III, which can be safely treated by definitive radiation therapy (10). Therapy for SCLC included active treatments, such as, chemotherapy, chemoradiotherapy (given sequentially or concurrently), thoracic radiotherapy, and supportive care only. Pulmonary function testing was performed using a bronchodilator in accordance with the protocol of the American Thoracic Society at time of lung cancer diagnosis. COPD was diagnosed when forced expiratory volume in 1 second was less than 70% of forced vital capacity (FEV1/FVC). Airflow limitation was classified as mild (GOLD I), moderate (GOLD II), or severe (GOLD III–IV) using the Global Initiative for Chronic Obstructive Lung Disease (GOLD) system (11).
The institutional review board of our hospital approved this retrospective study (IRB number-GBIRB2017-387) and the requirement for informed patient consent was waived.
CT-emphysema score
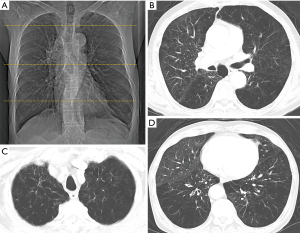
Chest CT scans were acquired on Siemens multi-detector helical CT scanners (SOMATOM Definition or SOMATOM Definition Flash, Siemens Healthcare, Forchheim, Germany). Patients were scanned in the supine position at full inspiration using the following technical parameters: 1 mm collimation, 120–140 kV, 75–350 mA, 0.75–1 s scan time, and 1–2 mm section thickness. A subspecialty-trained chest radiologist assessed emphysema severity according to the Goddard scoring system using CT images acquired at time of diagnosis (12). Each lung was divided into three areas, that is, upper, middle, and lower lung fields. An upper section was taken 1 cm above the superior margin of the aortic arch, a middle section 1 cm below the carina, and a lower section approximately 3 cm above the top of the diaphragm. Emphysema is characterized on CT images by low attenuation regions that contrast with surrounding normal lung parenchyma. Each area was graded using a 5-point scale, as follows; no emphysema (score 0), ≤25% emphysema (score 1), ≤50% emphysema (score 2), ≤75% emphysema (score 3) and >75% emphysema (score 4) (Figure 1). Scores from the six areas were summed to obtain a total score, which resulted in a possible minimum and maximum scores of 0 and 24, respectively. Because 95% of nonsmokers in a previous series had lungs with less than 5% emphysematous involvement (8), total CT-emphysema scores of 0 and 1 were considered to indicate the absence of emphysema.
Statistical analysis
The 149 study subjects were stratified based on the presence or absence of emphysema on CT, and these two groups were compared using Pearson’s chi-squared or Fisher’s exact test for categorical variables, or the Student’s t-test or the Mann-Whitney U test for continuous variables. Overall survival (OS) was estimated from date of baseline CT to death or last follow-up. Survival curves were estimated using the Kaplan-Meier method and differences were compared using the log-rank test. A maximal chi-square method available as open source statistical software (Maxstat; R Development Core Team, Vienna, Austria, http://www.R-project.org) was used to identify the optimal cutoff point for CT-emphysema score. Cox proportional hazard analysis was used to identify significant prognostic factors of survival. Variables with P values <0.05 by univariable analysis were included in the multivariable analysis, which was performed using the Enter method. Statistical significance was accepted for P values of <0.05. The analysis was performed using SPSS for Windows ver. 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of the study subjects and CT-emphysema scores
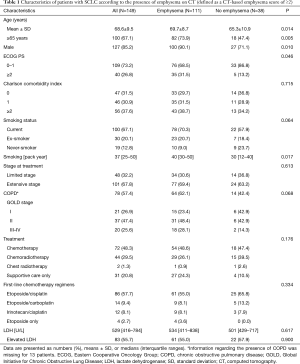
Baseline characteristics of the 149 consecutive study subjects are summarized in Table 1. Mean patient age was 68.6±9.5 years and 127 (85.2%) were men. One hundred and thirty patients (87.2%) had a history of smoking and 57.4% had COPD. Among all 149 study subjects, 101 (67.8%) had extensive stage at initial presentation, and 31 patients (20.8%) received supportive care only. Combination chemotherapy was administered to 112 (96.5%).
Full table
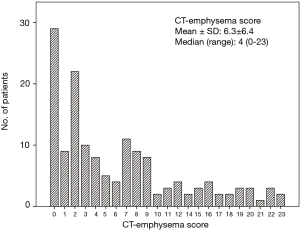
Emphysema was present in 111 patients (74.5%), and mean CT determined emphysema score was 6.3±6.4 (median, 4; range, 0–23) (Figure 2). Co-existent centrilobular and paraseptal emphysema was the most common type (72.9%), followed by centrilobular emphysema (17.1%) and paraseptal emphysema (9.9%). Emphysema was significantly associated with an older age (P=0.014), a male sex (P=0.010), and poorer PS (P=0.046). Patients with emphysema tended to have a smoking history (P=0.064) and had a significantly higher pack-year history (P=0.017). However, no significant differences were observed between patients with and without emphysema in terms of COPD prevalence, SCLC stage, treatment type, or serum lactate dehydrogenase level (LDH ≥486 U/L) (Table 1).
Survival analysis
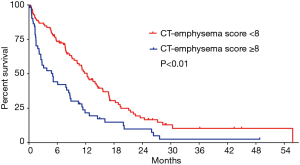
Over a median follow-up of 29.0 months [95% confidence interval (CI): 19.7–38.3 months], 123 patients (82.6%) died. Median OS for all study subjects was 9.6 months (95% CI: 7.2–11.9 months). Using optimal cut-off points determined using the maximal chi-square method, patients with a higher CT-emphysema score (≥8) showed significantly poorer OS (5.0 vs. 12.3 months) than those with lower CT-emphysema score (<7) (P<0.001 by the log-rank test) (Figure 3).
The results of univariable and multivariable analyses for prognostic factors of OS are given in Table 2. Univariable analysis showed an advanced age (P<0.001), poor PS (P<0.001), extensive stage (P<0.001), supportive care only (P<0.001), elevated LDH (P=0.006), and a higher CT-emphysema score (P<0.001), and the presence of COPD (P=0.011) were significantly associated with poor OS. Multivariable analysis revealed a higher CT-emphysema score [hazard ratio (HR), 1.03; 95% CI, 1.00–1.06; P=0.046], extensive stage (HR, 2.25; 95% CI, 1.44–3.53; P<0.001), and supportive care only (HR, 6.46; 95% CI, 3.64–11.48; P<0.001) independently predicted poor OS. The prognostic significance of advanced age, poor PS, elevated LDH, and COPD as determined by univariable analysis disappeared in the multivariable analysis.

Full table
Discussion
Approximately two-thirds of patients with SCLC present with clinically obvious extensive-stage disease that required systemic therapies (1). SCLC is known to be strongly associated with smoking history, which is also a major cause of COPD, or emphysema (13). Furthermore, lung cancer and emphysema share genetic susceptibility (14,15), and the association between the two appears to be associated with histologic type. A recent study showed a significantly higher risk of SCLC and squamous cell carcinoma in the presence of CT-detected emphysema than in other histologic types (16). Although the contribution made by emphysema to the risk of SCLC is recognized, no previous study has examined the prognostic impact of emphysema in patients with SCLC. In the present study, we found CT-emphysema score was a significant prognostic factor in SCLC, independently of other prognostic factors, such as, age, stage, PS, treatment, serum LDH, or COPD.
We observed a high prevalence of emphysema in patients with SCLC of 74.5% (based on a CT-emphysema score cut-off of ≥2), which is higher than those reported previously studies (5% for limited stage and 54% in all SCLC stages) (17). In our previous study on CT-emphysema scores in stage IIIB and IV squamous lung cancer (n=84), we found an emphysema prevalence of 71.4% and that a higher CT-emphysema score was a significant prognostic indicator in advanced squamous lung cancer (18).
COPD was not found to be an independent prognostic indicator in the present study. The prognostic significance of COPD in SCLC has not been studied, but previous studies have reported COPD had no prognostic impact in early-stage NSCLC or in patients with advanced NSCLC treated with chemotherapy (8,19). In addition, we found CT-emphysema score did not correlate well with FEV1(%), which concurs with a study by Ueda et al. (8). COPD is a heterogeneous syndrome, which consists of emphysema, chronic bronchitis, and small airway disease, and traditionally, its severity is assessed using spirometric parameters, such as, FEV1 (11).
Several mechanisms have been proposed for the association between emphysema and poor prognosis in lung cancer. First, genetic and epigenetic alterations (i.e., aberrant DNA methylation) are common in emphysema, which play a leading role in the modulation of prognosis in lung cancer (20). Another plausible mechanism is the reciprocal interactions between tumor microenvironments and clinical and pathologic aggressiveness of lung cancer (21). Matrix metalloproteinase (MMP)-3 is up-regulated in emphysema and has been demonstrated to play important roles in tumor progression (22). Moreover, emphysema has been also associated with poor performance and a risk factor of severe pneumonia (23,24). Although we found that poor PS and an advanced age were related to the presence of emphysema, they were not identified as independent prognostic indicators, which suggests that CT detected emphysema is a stronger prognostic effector than these factors.
In the present study, we used the Goddard semi-quantitative scoring method to assess the severity of emphysema at baseline on CT images. This visual scoring system is straightforward and can be performed quickly, because it does not require post-processing techniques, such as, segmentation, thresholding, and manual data extraction (25). Moreover, previous studies have reported good inter-observer agreements for semi-quantitative, 2D or 3D CT densitometry-based scoring systems used to assess the presence and extent of emphysema, and good correlations between subjective visual assessments and objective lung attenuation measurements (25,26). Since all lung cancer patients undergo a chest CT scan for diagnosis and staging at initial evaluation, this method enables rapid, reproducible measurement of emphysema severity without additional cost or radiation exposure.
The potential limitations of this study include its retrospective nature, the relatively small sample size, and the fact that it was conducted at a single institution. Furthermore, detailed information regarding the second-hand smoking or smoking status after cancer diagnosis cannot be obtained since this study was conducted in retrospective manner.
In conclusion, a higher emphysema score, as determined by baseline CT, was found to be associated with poor prognosis in SCLC, which indicates CT-based emphysema scoring systems might be used to better predict prognosis in SCLC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The institutional review board of our hospital approved this retrospective study (IRB number-GBIRB2017-387) and the requirement for informed patient consent was waived.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep 2012;61:1-51. [PubMed]
- Young RP, Hopkins RJ. How the genetics of lung cancer may overlap with COPD. Respirology 2011;16:1047-55. [Crossref] [PubMed]
- Madani A, Zanen J, de Maertelaer V, et al. Pulmonary emphysema: objective quantification at multi-detector row CT--comparison with macroscopic and microscopic morphometry. Radiology 2006;238:1036-43. [Crossref] [PubMed]
- Smith BM, Pinto L, Ezer N, et al. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer 2012;77:58-63. [Crossref] [PubMed]
- Smith BM, Schwartzman K, Kovacina B, et al. Lung cancer histologies associated with emphysema on computed tomography. Lung Cancer 2012;76:61-6. [Crossref] [PubMed]
- Gullon JA, Suarez I, Medina A, et al. Role of emphysema and airway obstruction in prognosis of lung cancer. Lung Cancer 2011;71:182-5. [Crossref] [PubMed]
- Ueda K, Jinbo M, Li TS, et al. Computed tomography-diagnosed emphysema, not airway obstruction, is associated with the prognostic outcome of early-stage lung cancer. Clin Cancer Res 2006;12:6730-6. [Crossref] [PubMed]
- Zulueta JJ, Wisnivesky JP, Henschke CI, et al. Emphysema Scores Predict Death From COPD and Lung Cancer. Chest 2012;141:1216-23. [Crossref] [PubMed]
- Kalemkerian GP, Gadgeel SM. Modern staging of small cell lung cancer. J Natl Compr Canc Netw 2013;11:99-104. [Crossref] [PubMed]
- Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256-76. [Crossref] [PubMed]
- Goddard PR, Nicholson EM, Laszlo G, et al. Computed tomography in pulmonary emphysema. Clin Radiol 1982;33:379-87. [Crossref] [PubMed]
- Jackman DM, Johnson BE. Small-cell lung cancer. Lancet 2005;366:1385-96. [Crossref] [PubMed]
- Adcock IM, Caramori G, Barnes PJ. Chronic obstructive pulmonary disease and lung cancer: new molecular insights. Respiration 2011;81:265-84. [Crossref] [PubMed]
- Punturieri A, Szabo E, Croxton TL, et al. Lung cancer and chronic obstructive pulmonary disease: needs and opportunities for integrated research. J Natl Cancer Inst 2009;101:554-9. [Crossref] [PubMed]
- Li Y, Swensen SJ, Karabekmez LG, et al. Effect of emphysema on lung cancer risk in smokers: a computed tomography-based assessment. Cancer Prev Res (Phila) 2011;4:43-50. [Crossref] [PubMed]
- Ytterstad E, Moe PC, Hjalmarsen A. COPD in primary lung cancer patients: prevalence and mortality. Int J Chron Obstruct Pulmon Dis 2016;11:625-36. [Crossref] [PubMed]
- Kim YS, Kim EY, Baek MY, et al. Prognostic Significance of CT Emphysema Score in Patients with Advanced Squamous Cell Carcinoma of the Lung. J Thorac Oncol 2015;10:S417-S.
- Izquierdo JL, Resano P, El Hachem A, et al. Impact of COPD in patients with lung cancer and advanced disease treated with chemotherapy and/or tyrosine kinase inhibitors. Int J Chron Obstruct Pulmon Dis 2014;9:1053-8. [Crossref] [PubMed]
- Gao YH, Guan WJ, Liu Q, et al. Impact of COPD and emphysema on survival of patients with lung cancer: A meta-analysis of observational studies. Respirology 2016;21:269-79. [Crossref] [PubMed]
- Murakami J, Ueda K, Sano F, et al. Pulmonary emphysema and tumor microenvironment in primary lung cancer. J Surg Res 2016;200:690-7. [Crossref] [PubMed]
- Michael M, Babic B, Khokha R, et al. Expression and prognostic significance of metalloproteinases and their tissue inhibitors in patients with small-cell lung cancer. J Clin Oncol 1999;17:1802-8. [Crossref] [PubMed]
- Eom JS, Song WJ, Yoo H, et al. Chronic obstructive pulmonary disease severity is associated with severe pneumonia. Ann Thorac Med 2015;10:105-11. [Crossref] [PubMed]
- Spruit MA, Watkins ML, Edwards LD, et al. Determinants of poor 6-min walking distance in patients with COPD: The ECLIPSE cohort. Respiratory Medicine 2010;104:849-57. [Crossref] [PubMed]
- Park KJ, Bergin CJ, Clausen JL. Quantitation of emphysema with three-dimensional CT densitometry: comparison with two-dimensional analysis, visual emphysema scores, and pulmonary function test results. Radiology 1999;211:541-7. [Crossref] [PubMed]
- Muller NL, Staples CA, Miller RR, et al. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest 1988;94:782-7. [PubMed]