Airway pressure release ventilation in patients with acute respiratory distress syndrome: not yet, we still need more data!
Airway pressure release ventilation (APRV) is a pressure-limited and time-cycled mode of mechanical ventilation that is without the need for patient-ventilator interaction. The original concept, first described by Downs and Stock in 1987, was to keep the patient at an elevated continuous positive airway pressure (CPAP) pressure most of the time, with periodic releases to facilitate CO2 clearance (1). Over the last 30 years, many studies of APRV have used a variety of different settings (Table 1) (7). This has caused confusion over what exactly an APRV mechanical breath profile is, and has contributed to the slow growth of evidence (7,8). Additionally, randomized controlled trials (RCTs) of APRV used control strategies that allowed tidal volumes that would not be considered lung protective by current standards (2-4). The lack of standard APRV settings, inconsistent use of lung protective ventilation in the comparator group, and the paucity of RCTs has led to appropriate skepticism about the true value (if any) of this mode.

Full table
There are four parameters (aside from FiO2) that need to be set when using APRV; pressure high (Phigh), pressure low (Plow), time high (Thigh; set by adjusting frequency on some ventilators), and time low (Tlow) (9). More recently, setting APRV has typically used an approach of maintaining Phigh for 90% of the ventilation time and adjusting Tlow during exhalation according to the expiratory flow pattern of the patient, to maintain an open-lung approach (with auto-PEEP) (8). Two RCTs have now used this approach as the basis for their APRV strategy, and they both used low tidal volume ventilation as their control strategy (5,6).
Since APRV is considered an open-lung approach that aims to increase mean airway pressure, minimize plateau pressure, promote lung recruitment, and improve oxygenation, it has been used and studied in patients with acute respiratory distress syndrome (ARDS). Despite this strong physiological rationale and numerous preclinical and clinical data, the study by Zhou et al. is the only RCT of APRV compared to low tidal volume ventilation in patients with ARDS (6). In this trial, 138 patients meeting ARDS criteria according to the Berlin Definition with a PaO2/FiO2 ≤250 mmHg were randomized to receive APRV or low tidal volume ventilation delivered by volume-assist control (10). They found significantly more ventilator free days (VFDs) at 28 days (19 vs. 2; P<0.001), and shorter ICU days (15 vs. 20; P=0.015) in the APRV group (6). ICU mortality was lower in the APRV group (19.7% vs. 34.3%; P=0.053), but this was not statistically significant. However, we feel the devil may be in the details.
There were differences between the APRV and control groups that were important, but seemed to be addressed by the authors. First, despite randomization the baseline characteristics were not evenly matched, with more patients with comorbidities randomized to the control group. Secondly, the sedation protocol allowed respiratory therapists to further titrate sedation and analgesia to achieve a minimum level of spontaneous ventilation between 30–60% of total minute ventilation in the APRV. As a result, there was significantly lower fentanyl and midazolam use in the APRV group, as well as lower sedation depth. The investigators attempted to address these concerns (i.e., imbalance in chronic diseases and sedation use) by performing a post hoc multivariable analysis in which APRV and sedation use were both independently associated with more VFDs.
For those less than enthusiastic over the current evidence for APRV the question still remains, how could APRV use result in more VFDs? One possible explanation is the limitation mentioned in the discussion by Zhou et al. that was not measured: patient-ventilator interaction.
Patient-ventilator interaction
One of the unique features of APRV, as already mentioned, is that it is pressure-limited and time-cycled without the need for patient-ventilator interaction. While asynchronies could exist at the moment of release, 90% of the time is spent at one elevated pressure, which by design allows minimal time for patient-ventilator asynchrony to occur. For the control group, Zhou et al. study used volume-assist control with inspiratory flow rates set close to 40 L/min and a decelerating flow pattern (as seen in the images provided in the electronic supplemental materials; ESM2). On the Puritan Bennet 840 ventilator (Covidien, Medtronic Inc. Minneapolis, MN) the set flow rate in volume-assist control is the maximum flow, that gradually slows down during inspiration when using a decelerating flow pattern. The results in longer inspiratory times compared to using constant flow. Longer inspiratory times can lead to delayed cycling, which can cause increased muscle workload, and ineffective efforts (11). Patients were switched to pressure support only to perform a spontaneous breathing trial (SBT), and if they failed, they were returned to the previous volume-assist control settings. When looking at the supplemental data (ESM2) there are two interesting findings. First, there are two ventilator screen images of a patient being managed with volume-assist control. The flow-time waveforms clearly show nearly half of the patient efforts are ineffective (Figure 1A,B). Secondly, the protocol (ESM1) dictates 6 mL/kg of predicted body weight (PBW) to be used, with the ability to increase tidal volume to 7 or 8 mL/kg if sufficient dyssynchrony is noted and plateau pressure is less than 30 cmH2O. By day three of the study, the mean (± standard deviation) of the set tidal volume was 7±1 mL/kg PBW (ESM2).
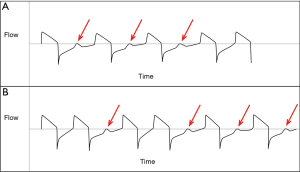
Asynchrony is associated with longer duration of mechanical ventilation, longer ICU stay, and increased ICU and hospital mortality (12-14). The final statement in the discussion by Zhou et al. mentions that patient-ventilator interaction was not measured, and that whether it could affect the outcomes would require further study.
Other considerations
In recent years, some sobering data has been published related to an open-lung approach. The Oscillation in Acute Respiratory Distress Syndrome (OSCAR) and Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) trials of high-frequency oscillatory ventilation (HFOV) found no benefit, and even harm, when HFOV was used early to manage patients with ARDS (15,16). Additionally, the recent Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) trial compared low-tidal volume and low-moderate PEEP to lung recruitment and PEEP set according to best respiratory system compliance (17). The study found significantly higher mortality using the open-lung approach of lung recruitment and compliance-based PEEP, and the VFDs were 0 in both groups. APRV is an open-lung approach that has not been tested to the extent that these other methods have, and particularly in light of these results, the potential efficacy of APRV requires confirmation in a large, rigorously conducted RCT.
Conclusions
We now have one small RCT of ARDS patients that compared APRV to low tidal volume ventilation. Although the study demonstrated improvements in a number of patient outcomes, there were a number of important limitations, including a control group that had a greater potential for patient-ventilator asynchrony that may have resulted in fewer VFDs and longer duration of ICU and hospital stay. Additionally, despite the fact that an open-lung approach has a strong physiological basis for its use based on animal data and small clinical trials, we have yet to see a large RCT demonstrate improvements in patient outcomes. Until then, we have ongoing equipoise regarding APRV, and do not recommend its routine use in patients with ARDS.
Acknowledgements
None.
Footnote
Conflicts of Interest: T Piraino has received payment for speaking and consultation from Philips, Drager, and Mallinckrodt. Dr. E Fan has received consultation fees from MC3 Cardiopulmonary, and is supported by a New Investigator Award from the Canadian Institutes of Health Research.
References
- Stock MC, Downs JB, Frolicher DA. Airway pressure release ventilation. Crit Care Med 1987;15:462-6. [Crossref] [PubMed]
- Putensen C, Zech S, Wrigge H, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 2001;164:43-9. [Crossref] [PubMed]
- Varpula T, Jousela I, Niemi R, et al. Combined effects of prone positioning and airway pressure release ventilation on gas exchange in patients with acute lung injury. Acta Anaesthesiol Scand 2003;47:516-24. [Crossref] [PubMed]
- Varpula T, Valta P, Niemi R, et al. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand 2004;48:722-31. [Crossref] [PubMed]
- Maxwell RA, Green JM, Waldrop J, et al. A randomized prospective trial of airway pressure release ventilation and low tidal volume ventilation in adult trauma patients with acute respiratory failure. J Trauma 2010;69:501-10; discussion 511. [Crossref] [PubMed]
- Zhou Y, Jin X, Lv Y, et al. Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive Care Med 2017;43:1648-59. [Crossref] [PubMed]
- Rose L, Hawkins M. Airway pressure release ventilation and biphasic positive airway pressure: a systematic review of definitional criteria. Intensive Care Med 2008;34:1766-73. [Crossref] [PubMed]
- Jain SV, Kollisch-Singule M, Sadowitz B, et al. The 30-year evolution of airway pressure release ventilation (APRV). Intensive Care Med Exp 2016;4:11. [Crossref] [PubMed]
- Habashi NM. Other approaches to open-lung ventilation: airway pressure release ventilation. Crit Care Med 2005;33:S228-40. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Gentile MA. Cycling of the Mechanical Ventilator Breath. Respir Care 2011;56:52-60. [Crossref] [PubMed]
- Thille AW, Rodriguez P, Cabello B. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 2006;32:1515-22. [Crossref] [PubMed]
- de Wit M, Miller KB, Green DA, et al. Ineffective triggering predicts increased duration of mechanical ventilation. Crit Care Med 2009;37:2740-5. [PubMed]
- Blanch L, Villagra A, Sales B, et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med 2015;41:633-41. [Crossref] [PubMed]
- Ferguson ND, Cook DJ, Guyatt GH, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013;368:795-805. [Crossref] [PubMed]
- Young D, Lamb SE, Shah S, et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013;368:806-13. [Crossref] [PubMed]
- Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators, Cavalcanti AB, Suzumura ÉA, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017;318:1335-45. [Crossref] [PubMed]


