Thoracoscopic wedge resection in single-lung patients
Introduction
Patients who have undergone pneumonectomy for lung cancer may develop new or metastatic cancer in the contralateral lung. Surgical resection for suspicious lung cancer on a single-lung patient after pneumonectomy for a previous primary lung neoplasm is an uncommon operation; slightly more than 100 cases have been reported in the literature (1). This challenging procedure is rarely indicated because of patients’ inadequate respiratory reserve or cancer spreading (synchronous distant metastasis). However, analysis of the published data suggests that pulmonary resection for metastatic or metachronous disease can be performed with acceptable morbidity and low mortality in appropriately selected patients (1). Recently, the thoracoscopic approach has become a standard procedure in the field of lung resection. However, its advantage in single-lung patients has not yet been well studied. In this report, we describe a series of successful thoracoscopic wedge resections for patients presenting with lung cancer after contralateral pneumonectomy.
Methods
From 2013 to 2015, 8 patients with a previous pneumonectomy (5 right and 3 left) for lung cancer underwent resection for a suspicious neoplasm on the remaining lung. Previous pneumonectomy was performed in all cases through posterolateral incision with systematic mediastinal lymphadenectomy. There were 5 males and 3 females of median age 63 years [interquartile range (IQR), 52–67 years]. We retrospectively reviewed the records of these patients. All lesions in the remaining lung were detected in the asymptomatic phase during regular follow-up based on repeated computer tomography (CT). Once the new nodule was discovered, preoperative examinations before surgery included laboratory tests, pulmonary function and exercise stress tests, echocardiography, bronchoscopy and 18-fluorodeoxyglucose positron emission tomography (PET) scan. Brain CT and/or bone scintigraphy were performed if necessary (in case of skeletal pains or neurologic symptoms). Patients were considered eligible for resection if their pulmonary function tests exceeded 40% of the predicted values [following the suggestions by Grodzki et al. (2)], Eastern Cooperative Oncology Group (ECOG) performance status was ‘0’ to ‘1’, and echocardiography did not indicate signs of pulmonary hypertension or right ventricle hypertrophy. Only single peripheral lesions of less than 2 cm were accepted for margin-free wedge resection, that is, the need of two or more wedge resections was an exclusion criterion. The new tumors were classified according to the Martini and Melamed criteria (3) as either metachronous lung cancer or metastatic lung cancer. The disease was staged according to the International Association for the Study of Lung Cancer (IASLC) seventh edition of TNM classification system of malignant tumors (4). The study obtained ethics approval by Comitato Etico per la Sperimentazione Clinica (CESC) della Provincia di Padova, Azienda Ospedaliera di Padova, Via Giustiniani 1, 35128 Padova (No./ID: 4346/AO/17).
Surgical procedure
At the time of the second surgery, two or three accesses were used to perform thoracoscopy. Anesthesia was based on single lumen tube intubation with fraction of inspired oxygen (FiO2) of 100% ventilation until hyperoxygenation was obtained, then the lung was kept in a deflated position for a few minutes. This allowed the surgeon to perform the first thoracoscopic incision in the most appropriate position, based on accurate CT evaluation, to identify the nodule and mark the corresponding visceral pleura with a dissector, and finally to plan the position of the second thoracoscopic incision. The procedure took a few minutes. At this point, all surgical instruments were removed from the thoracic cavity and the lung was inflated and ventilated with FiO2 100%. Then, the second and third incisions were completed, these had to be suitable to easily keep in position the marked area with endoscopic forceps. At this point the lung was hyper oxygenated again and submitted to a second deflation; the marked area was raised with thoracoscopic forceps from the second incision and wedge resection using surgical staplers was performed from optics or third access (if present). A single drain was placed in a standard manner to the apex from the mid-axillary line incision. During surgery, the level of oxyhemoglobin saturation was maintained above 90% to guarantee a sufficient tissue oxygenation. Its level was continuously monitored with peripheral pulse oximeter sensor and seriated arterial blood samples from a permanent intra-arterial catheter. Postoperative care was typical for wedge resection with special attention to potential respiratory and circulatory insufficiency. All patients were extubated at the operating theatre and intensively rehabilitated from the first few hours after surgery.
Results
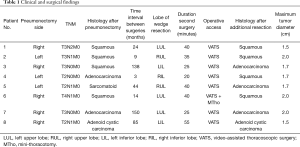
Table 1 reports the patients’ clinical data. All patients underwent wedge resection of less than single segment. The margins of resection were R0 in all patients. One patient needed an additional small thoracotomy incision because the margin of wedge resection performed through thoracoscopy was not adequate. Indeed, the resection margin was just a few millimeters from the neoplasm, so we decided to perform a wider resection with open surgery. Median total surgical operative time was 37.5 minutes (IQR, 25–40 minutes). There were no early postoperative deaths, while morbidity was 12.5%. One patient developed acute urine retention treated with urinary catheter application, no other post-operative complications were recorded.
Full table
All but one of the malignancies were metachronous, with a median interval between operations of 34 months (range, 3–150 months). Tumor type was adenocarcinoma in three patients, squamous cell carcinoma in four and adenoid cystic carcinoma in one. Three patients were considered to have a second primary bronchogenic carcinoma because of the different histologic type (when compared with that at pneumonectomy, in patients 3, 4, 5). A primary lung cancer was also considered in patient 7 and 8 according to the temporal criteria of Martini and Melamed. A metastasis of previous cancer was considered in cases 1, 2 and 6. Maximum tumor diameter was inferior or less than 2 cm (range, 1.5–2 cm).
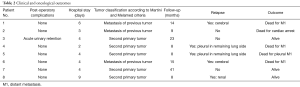
The median postoperative hospital stay was 4 days (IQR, 3.7–4.5 days). None of the patients developed severe chronic respiratory insufficiency after the operation, and all patients performed daily activities as before the first surgery. To date, two patients are still alive without evidence of disease and one is alive with renal metastasis treated with stereotactic radiotherapy. Four patients died of their disease (Table 2): two patients had cerebral recurrences, whereas two other patients had pleural recurrences. One patient died due to cardiac arrest. The overall median survival after redo surgery was 11.5 months (IQR, 8–17 months).
Full table
Discussion
The occurrence of a new lung nodule in patients who were previously submitted to pneumonectomy for lung cancer represents a challenge for thoracic surgeons. Optimal management of these patients is affected by a number of factors including the patients’ pulmonary reserve, associated medical comorbidity and clinical stage of the second lung cancer (5). In cases of limited disease, the therapeutic choice is usually balanced between surgical resection and non-surgical treatment. The percentage of patients with a potentially removable tumor that can tolerate additional resection is not well known. A recent literature review shows that pulmonary resection for metastatic or metachronous disease can be performed with acceptable morbidity (ranging from 6.2% to 28.6%) and low operative in appropriately selected patients who have previously undergone a pneumonectomy. Sub-lobar resection is the treatment of choice whenever possible, for which long-term results are rewarding especially for patients with metachronous lung cancer (1).
Available data on non-surgical treatment of the second tumor after pneumonectomy are even more limited. They included thermal ablation, radiofrequency ablation, conventional radiotherapy and stereotactic body radiotherapy. These techniques prove their major efficacy in small peripheral nodules and show a non-negligible risk of toxicity and local recurrences (6). Often non-surgical approaches are performed without any histological diagnosis of a lung nodule, because biopsy is considered too dangerous for single-lung patient. The absence of histological definition excludes the possibility to perform target therapy in compliant and suitable patients.
Lung surgery after pneumonectomy represents an anesthesiological and surgical challenge because the surgeon needs to work with a deflated lung, but there is the concomitant need to ensure adequate blood gas exchanges during the surgical procedure. To solve this problem, all reported cases so far provided a pulmonary resection through open surgery procedures with a semi-inflated lung; some authors also used cardio-pulmonary bypass to perform large resection with a deflated lung (1,7). However, extracorporeal circulation techniques are associated with a non-negligible risk of bleeding, paraplegia, stroke, cardiac events, respiratory or renal failure, and potential systemic spread of tumor cells, then they have to be especially avoided in oncological single-lung patients.
Surgery in single-lung patients can be justified if burdened with a low mortality and morbidity rate, and if it provides an advantageous outcome compared with non-surgical approaches. One of the potential key factors in reducing the risk for lung resection after contralateral pneumonectomy should be the minimal invasiveness of surgical maneuvers. Recent studies demonstrate better outcomes of minimal invasive thoracoscopic surgery versus open thoracotomy in patients with limited pulmonary function (8). Not using a rib spreader and limiting the damage to the inspiratory muscles led to low postoperative pain and better preservation of postoperative respiratory function. Thoracoscopic surgery is technically demanding in single-lung patients because it must be theoretically performed with a ventilated lung; the poor operatory field may make difficult to identify the lung nodule and increases the risk of lung damages, thus resulting in prolonged post-operative air leaks. A possible technical option is to use selective ventilation with bronchial blocker excluding the involved lobe, thus permitting an adequate operative field. Nakanishi et al. (9) and Fukui et al. (10) reported two cases of middle lobe lobectomy with the use of video-assisted thoracic surgery in a single-lung patient using selective ventilation of the right upper and lower lobes established by blocking the middle lobe branch with good results. Practically, this solution is feasible only in cases of middle lobe tumor, and in some cases of right superior lobe disease, due to inadequacy of the pulmonary reserve supplied by the ventilated lobes. Recuero Díaz et al. reported their experience on 12 patients treated with wedge resection in the remaining lung, one of which was performed using thoracoscopic approach (11). In line with this procedure, we propose to perform thoracoscopic surgery with a non-ventilated lung to gain an optimal operatory field and to avoid risks of lung damages. Apnea can be performed only for a few minutes, but in selected patients (good cardio-pulmonary reserve and small peripheral nodule) the time of surgery, following our operative protocol, is very short and can be performed in 2/3 times of apnea’s windows interspersed with recovery periods of hyper-oxygenation. Following this standardized and easily reproducible procedure, no intra or post-operative mortality or major complications occurred.
For a new lesion, suggestive of lung cancer in single-lung patients, the same tests as in the diagnosis of the initial cancer are performed. Patients are considered to be eligible for surgery in cases of single nodule without nodal involvement. It was found that the survival rate was better for metachronous than for metastatic cancer (12). In a wider cohort of patients, Ayub et al. (13) found that sublobar resection had higher median overall survival than did those who underwent lobectomy (42 vs. 18 months); median survival after resection for metachronous tumors was higher than after resection for metastatic cancers (40 vs. 28 months). This study lacks information about patients’ functional status, cardiopulmonary reserves, comorbidities, surgical approach (open vs. minimally invasive, type of thoracotomy) and reported at not negligible 1- and 3-month mortality (respectively, 11.1% and 12.7%).
Even if our study has many limitations, we noticed that the two patients still alive without relapse were those who had experienced the new lesion 138 and 150 months after the first tumor. Instead, second tumor histology did not influence survival. However, before denying a resection based on the belief that a metastasis could occur, it is necessary to consider that in most cases the histology of the new lesion cannot be determined before resection; a bronchoscopic biopsy is difficult to perform in peripheral nodules and fine needle CT guided biopsy can determine dangerous complications in single-lung patients that can affect the next chance to perform a surgical resection. If the diagnosis of metastasis cannot be established preoperatively, surgical resection is warranted. All resections we performed were wedge resections, which are usually considered inappropriate procedures for lung cancer (14,15). In these high-risk patients, limited resection seems to provide the best risk–benefit ratio compared to results obtained from non-surgical treatments. Histological definition, assured by a surgical procedure, gives patients the possibility to undergo further targeted therapies. Wedge resections were always performed obtaining a tumor free-margin removal. Surgery is not justified in case of centrolobar lesion that could result in a lobectomy or a deep wedge resection with the need of thoracotomic approach and with insufficient residual pulmonary function. Thus, broad pulmonary resections such as lobectomy, segmentectomy or multiple atypical resections have been associated with worse results in pneumonectomised patients (1,7,12). Furthermore, perihilar lesions can be easily biopsied during bronchoscopy. Considering these aspects and our results, thoracoscopic surgery should be considered as the initial approach, thoracotomy possibly needs to be subsequently performed only in case of suspicious of not margin-free removal or not clear nodule identification. The first event is avoidable with accurate pre-operative CT-imaging and selection criteria (with the exclusion of lung nodules that are too deep).
Conclusions
Our experience suggests that thoracoscopic surgery can be a feasible and advantageous surgical option compared with the thoracotomic approach in selected patients after contralateral pneumonectomy, with careful preoperative assessment and using short apnea windows in good collaboration with the anesthesiologists. Histological definition, made possible by the surgical procedure, gives patients the possibility to undergo further targeted therapies. Randomized prospective trials are necessary to assess the best management of peripheral small lung nodules in single-lung patients, and in particular to define which patients can benefit from a surgical approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study obtained ethics approval by Comitato Etico per la Sperimentazione Clinica (CESC) della Provincia di Padova, Azienda Ospedaliera di Padova, Via Giustiniani 1, 35128 Padova (No./ID: 4346/AO/17).
References
- Toufektziana L, Patris V, Potaris K, et al. Is it safe and worthwhile to perform pulmonary resection after contralateral pneumonectomy? Interact Cardiovasc Thorac Surg 2015;20:265-9. [Crossref] [PubMed]
- Grodzki T, Alchimowicz J, Kozak A, et al. Additional pulmonary resections after pneumonectomy: actual long-term survival and functional results. Eur J Cardiothorac Surg 2008;34:493-8. [Crossref] [PubMed]
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [PubMed]
- Sobin LH, Gospodarowicz MK, Wittekind C. editors. UICC TNM Classification of Malignant Tumours. 7th edition. New York: Wiley-Liss, 2009.
- Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst 1998;90:1335-45. [Crossref] [PubMed]
- Testolin A, Favretto MS, Cora S, et al. Stereotactic body radiation therapy for a new lung cancer arising after pneumonectomy: dosimetric evaluation and pulmonary toxicity. Br J Radiol 2015;88:20150228. [Crossref] [PubMed]
- Terzi A, Lonardoni A, Scanagatta P, et al. Lung resection forbronchogenic carcinoma after pneumonectomy: a safeand worthwhile procedure. Eur J Cardiothorac Surg 2004;25:456-9. [Crossref] [PubMed]
- Donahoe LL, de Valence M, Atenafu EG, et al. High Risk for Thoracotomy but not Thoracoscopic Lobectomy. Ann Thorac Surg 2017;103:1730-5. [Crossref] [PubMed]
- Nakanishi R, Hirai A, Muranaka K, et al. Successful videoassistedthoracic surgery lobectomy in a single-lung patient. Surg Laparosc Endosc Percutan Tech 2007;17:562-4. [Crossref] [PubMed]
- Fukui Y, Kohno T, Fujimori S, et al. Three-Port Thoracoscopic Middle Lobectomy in a Patient After Left Pneumonectomy. Ann Thorac Surg 2015;99:1422-5. [Crossref] [PubMed]
- Recuero Díaz JL, Rivas de Andrés JJ, Embún Flor R, et al. Outcomes of Pulmonary Resection in Single-Lung Patients. Cir Esp 2015;93:589-93. [PubMed]
- Donington JS, Miller DL, Rowland CC, et al. Subsequent pulmonary resection for bronchogenic carcinoma after pneumonectomy. Ann Thorac Surg 2002;74:154-8; discussion 158-9. [Crossref] [PubMed]
- Ayub A, Rehmani SS, Al-Ayoubi AM, et al. Pulmonary Resection for Second Lung Cancer After Pneumonectomy: A Population-Based Study. Ann Thorac Surg 2017;104:1131-7. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Miller DL, Rowland CM, Deschamps C, et al. Surgical treatment of non-small cell lung cancer 1 cm or less in diameter. Ann Thorac Surg 2002;73:1545-50; discussion 1550-1. [Crossref] [PubMed]


