Endobronchial treatment of peripheral tumors: ongoing development and perspectives
Introduction
As the capabilities of computed tomography (CT) scanners have progressed, higher levels of spatial resolution reveal tinier lung tumors. This situation has become a routine practice for the thorax specialists. Modern diagnostic tools, such as electromagnetic navigation (ENB) or radial probe endobronchial ultrasonography (radial EBUS) (1,2), offer more options for peripheral lung nodules diagnostic that are likely to be malignant (Figures 1,2). Moreover these tools combined with the recent possibility of bronchoscopic transparenchymatous biopsies open new diagnostic and therapeutic fields (3).
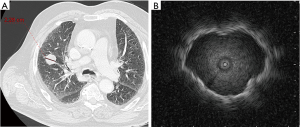
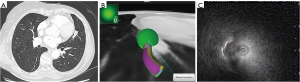
In the case of localized cancer, surgery remains the standard of care (4). When surgery is not possible, stereotactic radiotherapy (SBRT) is recommended (4). Percutaneous radiofrequency ablation (RFA) is an alternative treatment option (4,5). Stereotactic radiotherapy is expensive and includes radiation toxicities such as fibrosis, pneumonitis, cardiac toxicities and rib fractures. Percutaneous RFA has many adverse effects: pneumothoraces that may require chest tube insertion (22%) or even pleurodesis (1.6%), bleeding (1.6%), bronchopleural fistula (0.4%) and death (0.4%) (6).
The main advantage of ENB or radial EBUS over transparietal sampling is the low complication rate since no systematic puncture of the pleura is performed. Pneumothoraces occur in less than 10% (vs. more than 20% for CT-guided biopsies) and significant bleeding complications are very rare (vs. about 5% for transparietal sampling) (1,7). Also, endoscopic treatment of localized tumors seems an option to consider.
The endoscopic treatments of peripheral tumors without any node invasion raise some issues.
- The probe needs to be thin and flexible so that it can be inserted through a therapeutic endoscope;
- Good position of the therapeutic probe must be confirmed throughout the procedure by CT scan or fluoroscopy, eventually combined with EBUS;
- The area effect of the therapeutic probe needs to be known and should cover the whole tumor volume. In bulky tumors, multiple treatments can be necessary;
- Physical conditions of the therapeutic probe must be known such as a high temperature for RFA;
- Since these treatments are emerging with incomplete safety information, they should preferably be performed close to a surgical unit.
Bronchoscopy to guide RFA
After preclinical study on a sheep model (8), Tanabe et al. reported 10 cases of T1N0 lung cancer treated with various sizes and forms of RFA probes [5 mm, 8 mm (4 beads), 10 mm (5 beads)] (9). They used a cooled probe to prevent high temperature damages. Beads on the probe were used to enlarge the area of effect. The position of the probe was controlled by CT scan. They did not report any severe complication, only chest pain in 2 cases. The procedure was followed within 2 months by lung resection. With the largest probe, the tissue still contained few tumor cells at the border of the lesion. Histologic analysis indicated that the extent of the ablated area was 12 mm × 10 mm for the 10 mm probes. Then, 2 cases were published using the 10 mm (5 beads) probe in patients with a contraindication to surgery (10). No adverse effects were reported, and complete remission at 1 year was obtained in both cases. A third work from the same team was performed in 20 patients with clinical stage I cancers and a contraindication to surgery (11). They used their 10 mm cooled RFA probes on lung tumor (19–45 mm, without lymph nodes or metastasis involved). Control rate was 82% at 6 months (partial response 48%, stable disease 34%). Twelve lesions had to be retreated. Interestingly, 5-year overall survival was 61.5%. More recently, a team from Shanghai used ENB to guide RFA in 3 patients (12). No preclinical data were available. A bronchus sign, (defined by the visibility of a bronchus within the tumor on CT scan) was present for the 3 patients. Different probes were used with different area effect (15 to 30 mm). All 3 patients were in remission after 1 year. One patient has been retreated.
One prospective registry of ENB-guided RFA is currently active in clinicaltrials.gov (NCT03009630).
Bronchoscopy to guide radiotherapy
Brachytherapy
Brachytherapy is a highly localized radiation therapy. It has already been used for treatment of lung cancers of central airways (13). Only 2 studies have reported ENB-guided brachytherapy. It has been used to place a brachytherapy dedicated catheter in a few patients (14,15). Iridium 192 (15 to 30 Gy) was used in 10 patients and achieved complete remission. Nevertheless, compared to SBRT, this treatment seems to be less interesting for peripheral tumor. One prospective registry of ENB-guided brachytherapy is currently active in clinicaltrials.gov (NCT03051802).
To help stereotactic radiosurgery
Stereotactic radiotherapy may require fiducial markers to track respiration cycle and compensate for the changing tumor position (16), while reducing irradiation to the surrounding healthy lung. Fiducials can be inserted into intrathoracic tumors with different modalities: transthoracic, intravascular, and bronchoscopic (Figure 3). There are less pneumothoraces after bronchoscopy than after percutaneous placement (17). Nevertheless, there are alternative choices of tracking respiration which can reduce the indication for fiducial markers. The CYBERKNIFE® (Accuray, Sunnyvale, CA, USA) allows to not using fiducial with Xsight Spine Tracking System (18-20). Other machines may require using fiducial markers to reduce the radiotherapy field. The place to insert (within or around the tumor), and the number of fiducial markers (1 to 3 or more) to insert is not well established. The migration rate of some markers is very low (21).
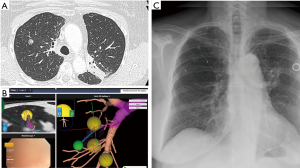
Cryotherapy
Cryotherapy uses intense cold to destroy tissue. Through freeze–thaw cycles, cryotherapy causes cell death and tissue necrosis. It can be used with a rigid or flexible bronchoscope. Traditional method uses a nitrous oxide enclosed cryoprobe with a metal tip for heat transfer. Cryotherapy has shown efficacy in central tumors (22). Percutaneous approach has also been reported (23). Transbronchial cryobiopsy is an emerging technique for the diagnosis of parenchymal disease such as infiltrative lung disease (24). However, there is no publication of bronchoscopic cryotherapy in peripheral lung cancer. The concerns related to the endoscopic approach are the penetration of cold delivery to parenchymal tumors.
Photodynamic therapy
Photodynamic therapy is based on local activation by laser light with a define spectrum after injection of a photosensitizer (25). It was originally used for superficial and proximal tumor in lung cancer. Preclinical data on a swine model has showed a 10mm necrotic area around the delivery light on normal lung (26). It was used percutaneously under CT-scan guidance in 9 patients with peripheral tumors, and 7 cases showed partial response. Several prospective registries of ENB-guided photodynamic therapy are currently active in clinicaltrials.gov (NCT03211078, NCT03344861, NCT02916745).
Conclusions
Evaluating these new diagnostic and therapeutic options requires a close partnership between thoracic surgeons, pulmonologists and R & D companies because of technical issues. Although safety signals are encouraging, more preclinical and post-treatment pathologic information is needed. Including patients in specific clinical trials should be prioritized.
Acknowledgements
None.
Footnote
Conflicts of Interest: The thoracic department received fees for presentation in a Medtronic symposium which were donated to our association.
References
- Makris D, Scherpereel A, Leroy S, et al. Electromagnetic navigation diagnostic bronchoscopy for small peripheral lung lesions. Eur Respir J 2007;29:1187-92. [Crossref] [PubMed]
- Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011;37:902-10. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Sterman D, et al. Bronchoscopic transparenchymal nodule access (BTPNA): first in human trial of a novel procedure for sampling solitary pulmonary nodules. Thorax 2015;70:326-32. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Chen H, Senan S, Nossent EJ, et al. Treatment-Related Toxicity in Patients with Early Stage Non-Small Cell Lung Cancer and Co-Existing Interstitial Lung Disease: A Systematic Review. Int J Radiat Oncol Biol Phys 2017;98:245-6. [Crossref]
- Kashima M, Yamakado K, Takaki H, et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center's experiences. AJR Am J Roentgenol 2011;197:W576-80. [Crossref] [PubMed]
- Laurent F, Michel P, Latrabe V, et al. Pneumothoraces and chest tube placement after CT-guided transthoracic lung biopsy using a coaxial technique: incidence and risk factors. AJR Am J Roentgenol 1999;172:1049-53. [Crossref] [PubMed]
- Tsushima K, Koizumi T, Tanabe T, et al. Bronchoscopy-guided radiofrequency ablation as a potential novel therapeutic tool. Eur Respir J 2007;29:1193-200. [Crossref] [PubMed]
- Tanabe T, Koizumi T, Tsushima K, et al. Comparative study of three different catheters for CT imaging-bronchoscopy-guided radiofrequency ablation as a potential and novel interventional therapy for lung cancer. Chest 2010;137:890-7. [Crossref] [PubMed]
- Koizumi T, Kobayashi T, Tanabe T, et al. Clinical experience of bronchoscopy-guided radiofrequency ablation for peripheral-type lung cancer. Case Rep Oncol Med 2013;2013:515160. [Crossref] [PubMed]
- Koizumi T, Tsushima K, Tanabe T, et al. Bronchoscopy-Guided Cooled Radiofrequency Ablation as a Novel Intervention Therapy for Peripheral Lung Cancer. Respiration 2015;90:47-55. [Crossref] [PubMed]
- Xie F, Zheng X, Xiao B, et al. Navigation Bronchoscopy-Guided Radiofrequency Ablation for Nonsurgical Peripheral Pulmonary Tumors. Respiration 2017;94:293-8. [Crossref] [PubMed]
- Skowronek J. Brachytherapy in the treatment of lung cancer - a valuable solution. J Contemp Brachytherapy 2015;7:297-311. [Crossref] [PubMed]
- Harms W, Krempien R, Grehn C, et al. Electromagnetically navigated brachytherapy as a new treatment option for peripheral pulmonary tumors. Strahlenther Onkol 2006;182:108-11. [Crossref] [PubMed]
- Becker HD, Harms W, Debus J, et al. Brachytherapy of inoperable peripheral lung cancer guided by electromagnetic navigation and endobronchial ultrasound: Feasibility study and confirmation by long-term results at two centres. Chest 2009;136: abstr 2.
- Anantham D, Feller-Kopman D, Shanmugham LN, et al. Electromagnetic navigation bronchoscopy-guided fiducial placement for robotic stereotactic radiosurgery of lung tumors: a feasibility study. Chest 2007;132:930-5. [Crossref] [PubMed]
- Schroeder C, Hejal R, Linden PA. Coil spring fiducial markers placed safely using navigation bronchoscopy in inoperable patients allows accurate delivery of CyberKnife stereotactic radiosurgery. J Thorac Cardiovasc Surg 2010;140:1137-42. [Crossref] [PubMed]
- Bibault JE, Prevost B, Dansin E, et al. Image-guided robotic stereotactic radiation therapy with fiducial-free tumor tracking for lung cancer. Radiat Oncol 2012;7:102. [Crossref] [PubMed]
- Senan S, Palma D. Stereotactic lung radiotherapy: do we need fiducial markers? Ann Thorac Surg 2011;91:335-6; author reply 336. [Crossref] [PubMed]
- Awano N, Ikushima S, Izumo T, et al. Efficacy and safety of stereotactic body radiotherapy using CyberKnife in Stage I primary lung tumor. Jpn J Clin Oncol 2017;47:969-75. [Crossref] [PubMed]
- Rong Y, Bazan JG, Sekhon A, et al. Minimal Inter-Fractional Fiducial Migration during Image-Guided Lung Stereotactic Body Radiotherapy Using SuperLock Nitinol Coil Fiducial Markers. PLoS One 2015;10:e0131945. [Crossref] [PubMed]
- Inaty H, Folch E, Berger R, et al. Unimodality and Multimodality Cryodebridement for Airway Obstruction. A Single-Center Experience with Safety and Efficacy. Ann Am Thorac Soc 2016;13:856-61. [Crossref] [PubMed]
- Zhang YS, Niu LZ, Zhan K, et al. Percutaneous imaging-guided cryoablation for lung cancer. J Thorac Dis 2016;8:S705-S9. [Crossref] [PubMed]
- Lentz RJ, Argento AC, Colby TV, et al. Transbronchial cryobiopsy for diffuse parenchymal lung disease: a state-of-the-art review of procedural techniques, current evidence, and future challenges. J Thorac Dis 2017;9:2186-203. [Crossref] [PubMed]
- Allison R, Moghissi K, Downie G, et al. Photodynamic therapy (PDT) for lung cancer. Photodiagnosis Photodyn Ther 2011;8:231-9. [Crossref] [PubMed]
- Kato H, Harada M, Ichinose S, et al. Photodynamic therapy (PDT) of lung cancer: experience of the Tokyo Medical University. Photodiagnosis Photodyn Ther 2004;1:49-55. [Crossref] [PubMed]


