Effects of pump speed changes on exercise capacity in patients supported with a left ventricular assist device—an overview
Introduction
As a disease affecting continually increasing patient numbers, heart failure (HF) remains a significant global health problem (1). The implantation of left ventricular assist devices (LVAD) has been successfully performed for treating terminal HF for several years (2-5).
Over the past few decades, a great deal of technical progress has been made in the field of LVAD therapy, leading to a reduction in the complication rate, as well as significant improvement in patient survival (6,7). The devices predominantly used today are continuous-flow pumps, which operate at a fixed rotational speed. The success of treatment with continuous-flow pumps is indisputable, with support durations of over 5 (up to 10) years now often being achieved (8,9). With treatments of such duration, regaining adequate exercise capacity becomes important. Existing literature however shows that exercise capacity following LVAD implantation remains considerably restricted (10-12). One possible cause could arise from the pump speed which remains unchanged during exercise, leading to resulting pump flow which cannot sufficiently adapt to haemodynamic changes (10). As the underlying disease also limits the output via the aortic valve, the total cardiac output (TCO) cannot be sufficiently increased. Consequentially the prerequisites for good exercise capacity are not met as they are in healthy persons (13).
In order to tackle this problem head on, automatic pump control has been an ongoing research field for several years now. One of the goals of automatic pump control is to increase TCO during exercise in order to deliver more oxygen for the muscles. To date, many strategies have been developed and evaluated both in silico and in vitro (14-18). Such algorithms have yet to find their way into routine use and this will probably also remain the case in the coming years. Their introduction is currently hindered, amongst other things, by the non-existence of reliable and robust sensors which could provide information about the filling pressure of the heart in high temporal resolution (17,18).
The current research focus is on whether, and to what extent, automatic pump control can positively influence exercise capacity, which parameters are altered as a result, and what level of improvement can be expected (19). Numerous studies have been published over the past few years in which pump speed has been manually adjusted before or during physical exercise, under medical supervision. The aim of this paper is to compare the results from these studies and to discuss the impact of pump speed adaptations on exercise capacity.
Review strategy
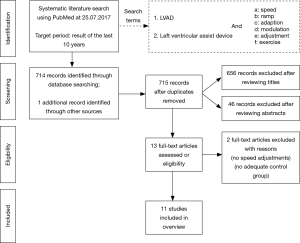
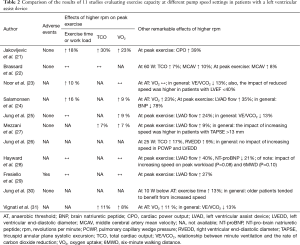
A systematic research of the literature was undertaken in PubMed using defined search criteria (Figure 1). Studies from the last 10 years which investigated the effect of at least two different pump speed settings on exercise capacity were considered. Ultimately, 11 relevant studies with a total of 161 patients could be included (21-31). Table 1 summarizes patient baseline characteristics, device type, time after implantation, as well as the exercise mode and the various pump speed settings. In order to evaluate exercise capacity, we took into consideration exercise time, peak work load, TCO, peak oxygen consumption (peak VO2) and, where available, values at the anaerobic threshold (AT). Possible complications were documented (Table 2).

Full table
Full table
Pump types and settings
Setting the LVAD pump speed (at rest) generally involves a compromise between suction prevention, optimal left-ventricular unloading and aortic valve opening. On one hand, sufficient TCO must be generated, but on the other possible complications (e.g., aortic valve fusion or arrhythmias) must be avoided (13,32,33).
The studies included used five different continuous-flow pumps in total. All devices had different rotational speed ranges (Table 3) and displayed individual pump characteristics (40). Shah et al. (41) recently reported that adapting the pump speed of the HeartMate II (HM II, Thoratec Corp., Pleasanton, CA, USA) by 400 rpm, for example, has the same effect on TCO (at rest) as adapting the HeartWare ventricular assist device (HVAD) device (HeartWare Inc., Framingham, MA, USA) by 130 rpm. Against this background, the speed adaptations performed in these studies must be evaluated individually. In the studies presented, four different types of pump speed were applied in total: reduced speed, baseline speed, increased speed and incremental increased speed during exercise. The magnitude of the speed adaptations differed in each case (Table 1).

Full table
Effects of pump speed adaptations
Safety and complications
A major problem when adapting pump speed is the risk of possible complications. In 5 out of the 11 studies, no short or long-term adverse events could be observed as a consequence of speed adaptations (21,22,25,27,30). Five other studies did not report any complications, but did not explicitly negate them either (23,24,26,28,31). Only Fresiello et al. (29) explicitly refer to observed complications: in their study, adverse events occurred in 2 out of 14 patients during incremental increased speed. In one case, non-sustained ventricular tachycardia was observed at maximum work load; in the other case, at the end of the examination the patient had a “hypotensive episode … due to a shift of the interventricular septum towards the left ventricle” (29).
Such data taken together suggests that increased speeds can incur risk. Overall, the LVAD speed was increased beyond the baseline level in 119 patients (from nine different studies); the two observed adverse events correspond to a percentage of 1.7%. However, if we take a look at the maximum pump speed in the study in question (HM II, 10,843 rpm) (29), we see that in two studies with comparable (HM II, 10,843 rpm) (25) or higher (HM II, 11,500 rpm) (22) pump speeds, no complications occurred.
There can be no doubt that increasing LVAD pump speed without any cardiac control parameters (e.g., intracardiac pressure values) remains difficult and risky. Nevertheless, in most cases manual speed adaptations could be performed safely, albeit without the maximum possible pump speeds ever being reached (Tables 1,3). Further investigation is required to test this hypothesis.
Work load, exercise time and six-minute walking distance (6MWD)
The peak work load or peak exercise time was observed in seven different studies: in 3 of them (21,23,24), increases between 10% and 18% could be achieved, in one study (28) a trend was observed (P=0.08). In the other three studies, authors (22,25,29) were unable to observe significant improvements. A look at the various pump speed types reveals that effects were only observed when reduced speed was compared with baseline or increased speed. Increases beyond baseline speed were unable to improve peak work load or peak exercise time significantly.
At submaximal level, the study by Hayward et al. (28) suggests a trend towards improvement in the 6MWD (P=0.10), although here, too, the effect only seems to be between reduced and baseline speed. Jung et al. (30), on the other hand, could achieve a 13% improvement in exercise time by changing from baseline speed to increased speed, with a constant work load just below AT.
TCO
TCO comprises LVAD flow and ejection through the aortic valve (11,13,26). The proportions can differ depending on the myocardial residual function, especially with increasing physical exercise (27,42). A change in pump speed can also shift the proportions (10,27,29). The combined overall quantity of blood which can be circulated through the body is crucial for exercise capacity.
Overall, 4 of the 11 studies measured TCO at peak exercise (21,22,27,31). In three studies, TCO could be significantly increased by means of a higher rotational speed (21,27,31). Comparable with workload and exercise time, the largest improvements could be achieved in conjunction with reduced speed vs. baseline speed (30%) (21). In contrast, increases beyond baseline speed achieved only little (7%) (27) or no effect (22).
TCO was observed at a submaximal level in two studies (22,26). Here, little to moderate effect (7% and 17%) could be observed. Notably, the study by Brassard et al. (22) was the only one to be conducted on a semi-supine ergometer cycle. Due to the deviating position of the body, the comparability of the TCO values in this study to those achieved on an upright ergometer cycle is difficult to estimate (43).
Oxygen consumption
VO2 at peak exercise was observed in 8 of the 11 studies. In five studies, VO2 could be significantly increased by means of a higher rotational speed (21,24,25,27,31). One study revealed a significant trend (28). The effects occurred independently of the initial pump speed (reduced or baseline) and were mostly within a range of 7–9%. In two studies no effects could be shown (23,29).
At a submaximal level VO2 was observed in three studies. In two of them, a significant increase in VO2 could be achieved at AT (11% and 23%) (24,31); in one study no effect could be seen (23).
Other effects
Speed adaptation affects not only TCO, but also blood pressure. At baseline speed, blood pressure was significantly higher at peak exercise compared with reduced speed. In combination with TCO, a 39% increase in the cardiac power index could be observed, and this is seen as a marker for cardiac performance (21).
By increasing the pump speed, Brassard et al. (22) observed an 8% improvement in cerebral perfusion at peak exercise (10% at submaximal exercise), compared with baseline speed.
Three further studies (23,25,31) found during cardiopulmonary exercise test (CPET) evidence for a 13% reduction in the relationship between minute ventilation and the rate of carbon dioxide reduction (VE/VCO2 slope), which is universally recognized as a prognostic mortality index for HF patients (44).
At the hormonal level, increasing pump speed (reduced speed vs. increased speed) leads to an observed decrease in brain natriuretic peptide (BNP) (78%) (24) and NT-pro-BNP (NT-proBNP) (21%) (28), an indication of significantly reduced pressure load in the cardiac atria.
Role of the residual myocardial function
The residual myocardial function of both ventricles generally plays an important role. Noor et al. (23) were able to prove that at reduced speed patients with a left-ventricular ejection fraction (LVEF) below 40% achieved a significantly poorer VO2 at peak exercise and a poorer VE/VCO2 slope during CPET; patients with an LVEF above 40% were able to compensate the reduced pump performance with their residual myocardial function. To date there has been no proven influence of LVEF when speed is increased beyond baseline.
The right-ventricular systolic function is also crucial (10,13). Through an increased pump speed and during physical exercise, venous reflux is considerably increased. There is a danger that the non-supported right ventricle is no longer fully capable of moving along the increased quantity of blood to the left ventricle (27,33). Mezzani et al. (27) found out that the only patients to profit from an increase in speed (improved peak VO2) were those who had a tricuspid annular plane systolic excursion (TAPSE) of >13 mm.
Muthiah et al. (45) report that the right ventricle can additionally suffer an overload when the left ventricle is not sufficiently unloaded (despite increased speed) and more blood accumulate. Further investigation is required to corroborate such data.
Clinical implications
The parameters TCO, VO2 and exercise time/work load are theoretically interdependent: through an increase in TCO, more oxygen can be transported through the circulatory system and ultimately be absorbed by the muscle (provided that oxidative capacity is sufficient). Through this increased supply of oxygen, more energy is made available to the muscle, ultimately leading to an increase in exercise time/work load (46,47).
Corresponding to these considerations, Table 2 shows that in most cases a study measured similar effects on the mentioned parameters. It was conspicuous that an acute reduction in pump speed below baseline speed led to considerable loss in exercise capacity (21,23,24,28). This particularly affects patients with a poor left-ventricular residual function who are unable to compensate the reduced pump flow (23). These results are in line with those of Camboni et al. (48), who found that an open aortic valve strategy leads to impaired exercise capacity and hemodynamics.
To date, increases in pump speed beyond baseline have had only little (25,27) or no (22,29) effect on exercise capacity. Fatigue and dyspnoea scores (28) as well as the rating of perceived exertion (30) remain unchanged. In one study, such increases even led to complications (29).
One possible reason for insufficient improvement in exercise capacity at increased speed is that LVAD patients often have an impaired right-ventricular systolic function (27,33). Some authors also report that pump speeds were probably not increased to a sufficient degree (22,28). A comparison between Table 1 and Table 3 shows, at least, that in no study was the rotational speed increased to its maximum level, although it is also true that this could not be expected for safety reasons.
In contrast to peak exercise, it was interesting that the effects of increasing pump speed at submaximal level were positive (22,24,26,30,31). This is very promising and should be pursued further since improved submaximal capacity (AT) is crucial to the mastering of everyday tasks (30) and can lead to improved patient participation.
Conclusions
In conclusion, it remains unclear whether or not pump speed is the crucial determining factor in the bid to increase exercise capacity (29). There are at least indications that physiological pump speed adaptation can contribute to improved submaximal exercise capacity which is crucial for activities of daily living. An increase in peak exercise, in contrast, seems to be only possible in some patients. However, research attempts at physiological pump speed adaptation are yet to be realized. In future, further studies will be required in order to improve our understanding of the special hemodynamics in LVAD patients under exercise conditions, and to identify those patients who could benefit from automatic pump speed control.
Acknowledgements
This project is funded by the German Federal Ministry of Education and Research (BMBF) within the framework of the ITEA 3 Project Medolution (14003).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Braunwald E.. The war against heart failure: the Lancet lecture. Lancet 2015;385:812-24. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Cvitkovic T, et al. First results of HeartWare left ventricular assist device implantation with tunnelling of the outflow graft through the transverse sinus. Interact Cardiovasc Thorac Surg 2017;25:503-8. [Crossref] [PubMed]
- Rojas SV, Avsar M, Hanke JS, et al. Minimally invasive ventricular assist device surgery. Artif Organs 2015;39:473-9. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015;34:1495-504. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Avsar M, et al. Minimally-invasive LVAD Implantation: State of the Art. Curr Cardiol Rev 2015;11:246-51. [Crossref] [PubMed]
- Slaughter MS. Chronic implantable mechanical circulatory support 50 years later: still shooting for the stars! Ann Thorac Surg 2015;99:749-51. [Crossref] [PubMed]
- Saeed O, Jorde UP. Advances in Continuous Flow Left Ventricular Assist Device Support for End-Stage Heart Failure: A Therapy in Evolution. Cardiol Rev 2017;25:84-8. [PubMed]
- Kirklin JK, Cantor R, Mohacsi P, et al. First Annual IMACS Report: A global International Society for Heart and Lung Transplantation Registry for Mechanical Circulatory Support. J Heart Lung Transplant 2016;35:407-12. [Crossref] [PubMed]
- Schmitto JD, Hanke JS, Rojas S, et al. Circulatory support exceeding five years with a continuous-flow left ventricular assist device for advanced heart failure patients. J Cardiothorac Surg 2015;10:107. [Crossref] [PubMed]
- Jung MH, Gustafsson F. Exercise in heart failure patients supported with a left ventricular assist device. J Heart Lung Transplant 2015;34:489-96. [Crossref] [PubMed]
- Loyaga-Rendon RY, Plaisance EP, Arena R, et al. Exercise physiology, testing, and training in patients supported by a left ventricular assist device. J Heart Lung Transplant 2015;34:1005-16. [Crossref] [PubMed]
- Schmidt T, Bjarnason-Wehrens B, Bartsch P, et al. Exercise Capacity and Functional Performance in Heart Failure Patients Supported by a Left Ventricular Assist Device at Discharge From Inpatient Rehabilitation. Artif Organs 2018;42:22-30. [Crossref] [PubMed]
- Reiss N, Schmidt T, Workowski A, et al. Physical capacity in LVAD patients: hemodynamic principles, diagnostic tools and training control. Int J Artif Organs 2016;39:451-9. [Crossref] [PubMed]
- Zimpfer D, Strueber M, Aigner P, et al. Evaluation of the HeartWare ventricular assist device Lavare cycle in a particle image velocimetry model and in clinical practice. Eur J Cardiothorac Surg 2016;50:839-48. [Crossref] [PubMed]
- Schmitto JD, Burkhoff D, Avsar M, et al. Two axial-flow Synergy Micro-Pumps as a biventricular assist device in an ovine animal model. J Heart Lung Transplant 2012;31:1223-9. [Crossref] [PubMed]
- Schmidt T, Reiss N, Deniz E, et al. Adaptive Pump Speed Algorithms to Improve Exercise Capacity in Patients Supported with a Left-Ventricular Assist Device. Stud Health Technol Inform 2017;236:235-40. [PubMed]
- AlOmari AH, Savkin AV, Stevens M, et al. Developments in control systems for rotary left ventricular assist devices for heart failure patients: a review. Physiol Meas 2013;34:R1-27. [Crossref] [PubMed]
- Bozkurt S.. Physiologic outcome of varying speed rotary blood pump support algorithms: a review study. Australas Phys Eng Sci Med 2016;39:13-28. [Crossref] [PubMed]
- Uriel N, Adatya S, Malý J, et al. Clinical hemodynamic evaluation of patients implanted with a fully magnetically levitated left ventricular assist device (HeartMate 3). J Heart Lung Transplant 2017;36:28-35. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [Crossref] [PubMed]
- Jakovljevic DG, George RS, Nunan D, et al. The impact of acute reduction of continuous-flow left ventricular assist device support on cardiac and exercise performance. Heart 2010;96:1390-5. [Crossref] [PubMed]
- Brassard P, Jensen AS, Nordsborg N, et al. Central and peripheral blood flow during exercise with a continuous-flow left ventricular assist device: constant versus increasing pump speed: a pilot study. Circ Heart Fail 2011;4:554-60. [Crossref] [PubMed]
- Noor MR, Bowles C, Banner NR. Relationship between pump speed and exercise capacity during HeartMate II left ventricular assist device support: influence of residual left ventricular function. Eur J Heart Fail 2012;14:613-20. [Crossref] [PubMed]
- Salamonsen RF, Pellegrino V, Fraser JF, et al. Exercise studies in patients with rotary blood pumps: cause, effects, and implications for starling-like control of changes in pump flow. Artif Organs 2013;37:695-703. [Crossref] [PubMed]
- Jung MH, Hansen PB, Sander K, et al. Effect of increasing pump speed during exercise on peak oxygen uptake in heart failure patients supported with a continuous-flow left ventricular assist device. A double-blind randomized study. Eur J Heart Fail 2014;16:403-8. [Crossref] [PubMed]
- Jung MH, Hassager C, Balling L, et al. Relation between pressure and volume unloading during ramp testing in patients supported with a continuous-flow left ventricular assist device. ASAIO J 2015;61:307-12. [Crossref] [PubMed]
- Mezzani A, Pistono M, Corrà U, et al. Systemic perfusion at peak incremental exercise in left ventricular assist device recipients: Partitioning pump and native left ventricle relative contribution. IJC Heart & Vessels 2014;4:40-5. [Crossref]
- Hayward CS, Salamonsen R, Keogh AM, et al. Impact of left ventricular assist device speed adjustment on exercise tolerance and markers of wall stress. Int J Artif Organs 2015;38:501-7. [Crossref] [PubMed]
- Fresiello L, Buys R, Timmermans P, et al. Exercise capacity in ventricular assist device patients: clinical relevance of pump speed and power. Eur J Cardiothorac Surg 2016;50:752-7. [Crossref] [PubMed]
- Jung MH, Houston B, Russell SD, et al. Pump speed modulations and sub-maximal exercise tolerance in left ventricular assist device recipients: A double-blind, randomized trial. J Heart Lung Transplant 2017;36:36-41. [Crossref] [PubMed]
- Vignati C, Apostolo A, Cattadori G, et al. Lvad pump speed increase is associated with increased peak exercise cardiac output and vo2, postponed anaerobic threshold and improved ventilatory efficiency. Int J Cardiol 2017;230:28-32. [Crossref] [PubMed]
- Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant 2010;29:S1-39. [Crossref] [PubMed]
- Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157-87. [Crossref] [PubMed]
- HeartMate II LVAS Left Ventricular Assist System: Operating Manual Available online https://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4333b2-18-%209_2%20HM%20II%20Operating%20Manual.pdf
- HeartWare Inc. HeartWare® Ventricular Assist System: Instructions for Use. Available online: http://www.heartware.com/sites/default/files/uploads/docs/ifu00001_rev_15.pdf
- Berlin Heart GmbH. INCOR® Superior Pump Implantable Ventricular Assist Device: Patient's Instructions for Use. Edition 6. Available online: http://www.berlinheart.de/UserFiles/Downloaddokumente/Medical_Professionals_Distributoren/Non_US/INCOR_Implantierbares_LVAD_System/Gebrauchsanweisungen_und_zugehoerige_aktuelle_Mitteilungen/Patient5000017x04Rev60AM5000120x13en.pdf
- Schmid C, Tjan TD, Etz C, et al. First clinical experience with the Incor left ventricular assist device. J Heart Lung Transplant 2005;24:1188-94. [Crossref] [PubMed]
- Jarvik Heart Inc. Operator Manual Jarvik 2000® Adult Ventricular Assist System, Post-Auricular Cable. Available online: http://www.perfusion.ws/documents/Jarvick/JarvickOpManual.pdf
- Esmore D, Spratt P, Larbalestier R, et al. VentrAssist left ventricular assist device: clinical trial results and Clinical Development Plan update. Eur J Cardiothorac Surg 2007;32:735-44. [Crossref] [PubMed]
- Moazami N, Fukamachi K, Kobayashi M, et al. Axial and centrifugal continuous-flow rotary pumps: a translation from pump mechanics to clinical practice. J Heart Lung Transplant 2013;32:1-11. [Crossref] [PubMed]
- Shah P, Badoe N, Phillips S, et al. Unrecognized Left Heart Failure in LVAD Recipients: The Role of Routine Invasive Hemodynamic Testing. ASAIO J 2018;64:183-90. [Crossref] [PubMed]
- Jacquet L, Vancaenegem O, Pasquet A, et al. Exercise capacity in patients supported with rotary blood pumps is improved by a spontaneous increase of pump flow at constant pump speed and by a rise in native cardiac output. Artif Organs 2011;35:682-90. [Crossref] [PubMed]
- Muthiah K, Gupta S, Otton J, et al. Body position and activity, but not heart rate, affect pump flows in patients with continuous-flow left ventricular assist devices. JACC Heart Fail 2014;2:323-30. [Crossref] [PubMed]
- Forman DE, Myers J, Lavie CJ, et al. Cardiopulmonary exercise testing: relevant but underused. Postgrad Med 2010;122:68-86. [Crossref] [PubMed]
- Muthiah K, Robson D, Prichard R, et al. Effect of exercise and pump speed modulation on invasive hemodynamics in patients with centrifugal continuous-flow left ventricular assist devices. J Heart Lung Transplant 2015;34:522-9. [Crossref] [PubMed]
- Brooks GA, Fahey TD, Baldwin KM. Exercise physiology: Human bioenergetics and its applications. 4th edition. Boston: McGraw-Hill, 2005.
- Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 2015;95:549-601. [Crossref] [PubMed]
- Camboni D, Lange TJ, Ganslmeier P, et al. Left ventricular support adjustment to aortic valve opening with analysis of exercise capacity. J Cardiothorac Surg 2014;9:93. [Crossref] [PubMed]


