Immune checkpoint blockade for advanced non-small cell lung cancer: challenging clinical scenarios
Introduction
Immune checkpoint blockade has shown anti-tumour activity and improved survival in advanced non-small cell lung cancer (NSCLC). Both anti-PD-1 (nivolumab, pembrolizumab) and anti-PD-L1 monoclonal antibody (atezolizumab, durvalumab, avelumab) have been evaluated in metastatic NSCLC. Nivolumab, pembrolizumab and atezolizumab are currently approved for use in clinical practice due to demonstrated improvement in response rate, overall survival (OS) and quality of life (QoL) over standard chemotherapy. Anti-CTLA-4 agents such as ipilimumab and tremelimumab are currently being investigated, alone or in combination in a number of clinical trials; and it is likely these agents will be integrated into clinical practice over the next few years.
We present a series of case studies that highlight clinical challenges that these novel agents present. Through these cases we will review rare immune-related adverse events (AEs), optimal treatment duration and patient selection. We will also address real-life clinical scenarios such as treatment re-challenge and management of immune-related AEs.
Case 1: rare immune-related toxicities secondary to checkpoint inhibitors
A 74-year-old man with no prior history of autoimmune disease was diagnosed with stage 4 non-small cell lung adenocarcinoma with metastatic pleural nodules (cT2bN0M1a) at diagnosis. Mutation in KRAS variant c.35G>T (p.Gly12Val) was identified with no mutations in EGFR/ALK/ROS1 found. Past medical history included hypertension, macular degeneration and benign prostatic hypertrophy. The patient completed 4 cycles of cisplatin/pemetrexed followed by maintenance pemetrexed. After 4 cycles of maintenance pemetrexed, he progressed with bilateral lung metastasis and subsequently received treatment with docetaxel +/− selumetinib in a clinical trial (NCT01933932). After 9 months, his disease progressed again with enlarging lung metastases.
As part of a clinical trial (NCT02087423) his tumor sample was tested for PD-L1 expression (Roche Ventana SP263 assay) and received durvalumab 10 mg/kg every 2 weeks. Best response to treatment was stable disease confirmed after 4 cycles. After 18 cycles, the patient was admitted for G3 fatigue and G3 loss of appetite. A hormone panel revealed critically low (<50 nmol/L) serum cortisol associated with hyponatremia (Na 121 mmol/L, normal range 135–145 mmol/L). Renal function, serum potassium, thyroid function, testosterone and ACTH levels were all normal. Pituitary MRI scan excluded evidence of hypophysitis. A restaging CT scan excluded presence of adrenal metastasis and confirmed disease stability. The patient was diagnosed with adrenal insufficiency secondary to durvalumab. High-dose prednisolone (1 mg/kg/day) was commenced and switched to hydrocortisone after 48 hours with prompt resolution of fatigue and poor appetite in this timeframe. He was discharged with a tapering dose of hydrocortisone replacement until he reached a maintenance dose of 20 mg hydrocortisone daily in divided doses.
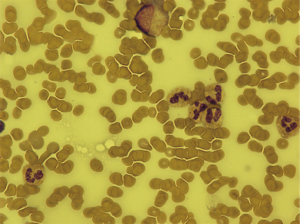
The patient continued on durvalumab with sustained stable disease for a further 4 months until he presented with G3 thrombocytopenia [platelets 31×109/L, normal range (150–400) ×109/L]. Bone marrow biopsy showed alternative hypocellular and monocellular areas with overall orderly trilineage hematopoiesis excluding marrow infiltration or failure as the cause of thrombocytopenia (Figure 1). No drug precipitants were identified. Repeated viral serology was negative. In the absence of any other causality, the patient was diagnosed with immune-mediated thrombocytopenia secondary to durvalumab. Durvalumab was withheld and prednisolone 1 mg/kg/day commenced. Return to normal platelet count occurred within 7 days (Figure 2). Once platelet count normalised, prednisolone was reduced by 10 mg per week over an 8-week period before switching back to maintenance hydrocortisone. The patient was re-challenged with durvalumab and, after only one further cycle, he presented with G4 thrombocytopenia (platelet 22×109/L). At this stage, high dose steroids were re-started and durvalumab was permanently discontinued.

Whilst tapering prednisolone for immune-mediated thrombocytopenia for the second time, the patient reported epigastric pain without loose or more frequent bowel motions. Review of routine restaging imaging revealed radiological evidence of colitis. Subsequently, corticosteroids were increased from 20 to 80 mg daily (equivalent 1 mg/kg daily) and gradually tapered over 8 weeks for treatment of colitis. This led to resolution of colitis after 10 weeks of steroid therapy.
At present, the patient remains on clinical and radiological follow-up with sustained stable disease after 6 months since durvalumab discontinuation.
Case discussion
With the increasing number of advanced lung cancer patients being treated with immune checkpoint inhibitors in various lines, the importance of identifying and initiating prompt treatment of immune related AEs (irAEs) is paramount. irAEs can affect any organ system and can vary in onset and severity in each individual.
Rates of irAEs between PD-1 and PD-L1 agents are similar (1). Safety data on metastatic NSCLC patients show that the most common irAEs across all PD-1/PD-L1 agents are rash, fatigue, thyroid dysfunction and immune-related hepatitis (2). Permanent discontinuation following toxicity occurred in 7.1% and 10% of previously untreated advanced NSCLC patients treated with pembrolizumab, and nivolumab respectively (3,4). Atezolizumab discontinuation due to toxicity occurred in 7% of NSCLC patients (5). Checkmate 012 demonstrated that irAEs occur with greater frequency and severity with combination CTLA-4/PD-1 therapy (6). Combination ipilimumab/nivolumab resulted in 38–45% incidence of grades 1–2 and 33–37% incidence of grades 3–4 irAE events. Much higher rates of colitis (23%) was observed and attributed to ipilimumab.
Familiarity with both common and rare complications of immunotherapy is vital given the expanded use of these agents as a standard of care in metastatic lung cancer. Diagnosis and treatment of common irAEs have clear management algorithms (7). Rarer neurological, cardiac, renal, haematological, ocular and rheumatological irAEs have been described.
This section will expand on a number of rare irAEs the clinician may encounter. The overall incidence of these events may be grossly underestimated due to under-reporting or misdiagnosis of these AEs.
Neurological toxicity
A spectrum of immune-related neurological events have been described secondary to immunotherapy including polyneuropathy, facial nerve palsy, demyelination, Guillain-Barré syndrome, myasthenia gravis, posterior reversible leukoencephalopathy, enteric neuropathy transverse myelitis, aseptic meningitis and encephalitis (7). A combined analysis of 59 immunotherapy trials observed a 6.1% incidence of neurological toxicity secondary to anti-PD-1 agents and up to 12% with combination therapy (8). Median time to onset was 6 weeks. Most of these events were low grade consisting of non-specific symptoms such as headache. High grade serious neurological irAEs account of <1% of cases. Lumbar puncture should be performed if there is a clinical suspicious of encephalopathy, meningitis, meningoradiculitis or Guillain-Barré like syndromes. Elevated CSF protein supports the diagnosis of an inflammatory mediated condition. Presence of auto-antibodies in immunotherapy-related events is rarely found. In cases of severe neurological toxicity, high dose steroids treatment should be started and treatment should be permanently discontinued.
Cardiac toxicity
Immune-mediated cardiac toxicity may manifest as myocarditis, cardiomyopathy or myocardial fibrosis (9). Incidence of immune-mediated myocarditis was reported to be 0.06% for single agent nivolumab and 0.27% for combination nivolumab/ipilimumab (10). Clinical presentation is highly variable ranging from subclinical disease to chest pain, cardiac failure, cardiogenic shock, arrhythmias and sudden death. Immune-mediated myocarditis is associated with a short median time to onset of 17 days from first infusion. Initial treatment is high dose corticosteroids (7). Most cases of immune-mediated cardiac toxicity have been fatal. One published case of steroid-refractory immune-mediated cardiac toxicity has responded to anti-thymocyte globulin (ATG) therapy (11). Close consultation with a cardiologist is advised.
Haematological toxicity
Haematological toxicities such as thrombocytopenic purpura, autoimmune haemolytic anaemia and lethal aplastic anaemia are rare but reported in the literature (12-14). High dose corticosteroid therapy should be promptly initiated and should be managed similarly to most common irAEs.
Case 2 immune-related toxicity after discontinuation of checkpoint inhibitors
A 65-year-old female with a past medical history of chronic obstructive pulmonary disease (COPD), osteoarthritis and stable hypothyroidism was diagnosed with stage IIIA (pT3pN1M0) NSCLC of large cell histology, EGFR/ALK/ROS1 wild-type. She underwent a left pneumonectomy followed by 4 cycles of adjuvant cisplatin/vinorelbine. Eighteen months later, she relapsed with local recurrence and new mediastinal lymphadenopathy. She completed 4 cycles palliative carboplatin/gemcitabine however progressed after 6 months. PD-L1 expression (Dako 22C3) on tumour sample was >1% and was commenced on pembrolizumab 2 mg/kg every 3 weeks. She developed progressive disease after 8 months on pembrolizumab and treatment was stopped.
Four weeks after last pembrolizumab dose, she presented with G3 liver transaminase elevation: AST 171 IU/L (ULN 33 IU/L) and ALT 263 IU/L (ULN 49 IU/L). Bilirubin remained within normal range. Hepatitis serology was negative. No other drug precipitants were identified. Liver metastases were excluded on imaging. She was diagnosed with immune-mediated hepatitis and commenced on dexamethasone 6 mg b.d. (equivalent dose of prednisolone 1 mg/kg/day). Steroid were tapered to dexamethasone 4 mg b.d., however within one week, her AST and ALT started to rise again. Dexamethasone was kept at 4 mg b.d. for a further 2 weeks before slowly tapering over 4 weeks and this led to return to normal liver function.
A tier 3 RAS variant was subsequently identified with next generation sequencing and the patient was enrolled onto a clinical trial to receive palbociclib (NCT02664935). After 12 weeks of treatment, she currently has stable disease.
Case discussion
While most irAEs have a median time to onset of 6–12 weeks after initiation of therapy (15,16), onset of first irAE has been documented as late as 12 months after treatment discontinuation (7). Onset and resolution of irAE has been extensively studied in single agent ipilimumab. A characteristic time course has been observed whereby median time to onset for skin toxicity is 3 weeks, hepatic toxicity 3–9 weeks, gastrointestinal toxicity 8 weeks and endocrinopathies 7–20 weeks (17). Prompt treatment with corticosteroids for grade 2 and above events usually results in resolution within 12 weeks for most irAEs. Data on time to onset of irAEs has been reported for single agent nivolumab in a melanoma population. In this cohort, median time to onset of irAE was 8 weeks for pulmonary toxicity, 6 weeks for endocrinopathies and 13 weeks for hepatic toxicity (18).
Various responsible mechanisms for late onset irAEs have been proposed. One possible mechanism in nivolumab-treated patients is its sustained binding to PD-1 on circulating T cells. Although the frequency of PD-1 occupancy by nivolumab has been shown to decrease after treatment discontinuation, binding to PD-1 was still detected for more than 20 weeks after cessation of treatment (19). T-cell memory may also have a role with potential for recognition of self-antigens even after cessation of checkpoint blockade (20).
Whilst time to onset of irAE has not been clearly implicated in survival outcomes, a small prospective study showed that early onset of irAE (<6 weeks from treatment initiation) was associated with higher objective response rates and progression free survival (21). Results of larger prospective studies are needed.
Case 3: sustained response after cessation of immunotherapy for immune related toxicity
A 57-year-old female was diagnosed with stage IV (T2aN1M1c) lung adenocarcinoma, EGFR-wild type, ALK/ROS1 negative. Her past medical history included only COPD. She was enrolled in a first-line clinical trial (NCT02542293) and received combination durvalumab and tremelimumab. After 2 cycles she achieved partial response with reduction in size of the left sided primary lesion and liver metastases.
After 3 cycles of treatment she presented with G2 hypothyroidism with an elevated TSH (102.9 mU/L, normal range 0.55–4.78 mU/L) and low free T4 (6.9 pmol/L, normal range 10–11 pmol/L). Levothyroxine 100 mcg daily was commenced and immunotherapy treatment continued.
After 4 cycles of combination treatment, she presented with headache, fatigue, nausea and vomiting. High dose prednisolone (1 mg/kg/day) was initiated for suspected hypophysitis. This diagnosis was confirmed with pituitary MRI scan demonstrating an enlarged anterior pituitary gland without evidence of a discrete mass and decreased pituitary hormone levels such as: TSH 0.02 mU/L (normal range 0.55–4.78 mU/L), cortisol <50 nmol/L and ACTH <5 ng/L (normal range 0–46 ng/L). Her symptoms rapidly responded to steroids and patient was then switched to a tapering dose of prednisolone over 6 weeks. Durvalumab and tremelimumab were permanently discontinued and patient remained on clinical and radiological follow up. Twelve months since discontinuation of immunotherapy, the patient remains in partial response and continues on replacement thyroxine and hydrocortisone therapy as ongoing treatment for immune-related endocrinopathies.
Case discussion
The ability to maintain tolerance to self-antigens and recognise foreign pathogens is in part maintained by inhibitory immune regulatory pathways, referred to as ‘immune checkpoints’ (22). By engaging immune checkpoints, tumors have the ability to evade immune surveillance resulting in uninhibited growth. The aim of agents that target the immune checkpoints, CTLA-4 and PD-1 and its ligand PD-L1, is to restore T-cell activation and immune tolerance to result in an anti-tumour response. Long-term sustained remissions have been demonstrated with both single agent and combination checkpoint inhibitors for treatment of melanoma (23,24). In lung cancer, more mature data is required.
Long-term survival data for nivolumab in pre-treated NSCLC patients within the CA209-003 phase 1b study indicates that sustained remissions can be achieved with a reported 5-year OS rate of 16% (25). Notably, in this study, nivolumab was administered for a maximum of 2 years. The results are also promising for other immunotherapy agents in the first-line and pre-treated settings, reporting longer duration of sustained anti-tumour response compared to conventional cytotoxic therapy. Sustained response has been observed in patients treated with first-line pembrolizumab and PD-L1 expression >50%, where the median duration of response has not yet been reached as reported in an updated 2-year follow-up analysis (26). In the second-line setting, median duration of response from nivolumab resulted was 17.2 months in non-squamous and not yet been reached (range, 2.9–20.5+ months) in squamous patients (27,28). In the only combination first-line trial published, the median duration of response has also not yet been reached in the 33 patients who have achieved an objective tumour response after a follow up period of 11.8–12.8 months (29).
Of most interest, is the emerging evidence that long-term survival from lung cancer can still be attained even if immunotherapy is discontinued due to toxicity. In the CA209-003 phase 1b study, 25% (n=4/16) of patients who lived >5 years had stopped nivolumab due to toxicity (25).
An analysis from the phase 3 OAK study of atezolizumab vs docetaxel after platinum therapy found overall that the development of an irAE did not negatively impact survival (30). Within the atezolizumab arm, incidence of irAE was 31% (G1-2 25%, G3-4 6.2%, no G5) with use of corticosteroids reported in 6%. OS was longer in patients with an irAE compared to patients without an irAE (HR 0.79, 95% CI: 0.6–1.05). The impact of corticosteroids and atezolizumab efficacy should be further explored as a difference was detected in median OS (16 vs. 21.9 months) in patients who received corticosteroids (n=24) vs. patients who did not (n=106), respectively.
Of interest, a retrospective analysis of 51 patients with advanced NSCLC treated with pembrolizumab found that OS was significantly longer in patients who developed immune-mediated thyroid dysfunction compared to patients who did not (HR 0.29, 95% CI: 0.09–0.94; P=0.04) (31). Retrospective data on 40 patients with advanced NSCLC treated by nivolumab found a positive correlation between development of skin irAEs and tumour response (32). Further data is required to define the association between incidence and severity of irAEs and its impact on survival.
Durable clinical benefit after cessation of immunotherapy due to toxicity has been observed (29). Histology and PD-L1 expression are not clearly predictive of long term survival. Onset and severity of irAEs, use of corticosteroids and its associations with survival, durability and depth of response needs further investigation. Emerging trials may give clearer insight into these issues.
Case 4: optimal treatment period and re-challenging in patients in responders
A 53-year-old female with a past history of early stage left breast cancer treated radically 13 year ago was diagnosed with stage IIIA (pT1bpN2M0) non-small cell lung adenocarcinoma, EGFR/ALK/ROS1 wild-type. She underwent left upper lobectomy followed by 4 cycles of adjuvant cisplatin/vinorelbine. Two years later, her lung cancer relapsed with mediastinal lymphadenopathy and bilateral lung metastases. She progressed after 4 cycles of first-line palliative cisplatin/pemetrexed and commenced on second-line erlotinib. After 4 months on erlotinib, her disease further progressed with new lung, nodal and solitary liver metastases.
She received third-line durvalumab 10 mg/kg every 2 weeks on a clinical trial (NCT02087423) and continued durvalumab for a total of 26 cycles (total planned treatment), maintaining stable disease before switching to observation per trial protocol. She remained on observation with 6-weekly CT scans for another 12 months until progression was detected due to increase in size of mediastinal lymphadenopathy. Durvalumab was recommenced as re-challenge was allowed per protocol. The patient currently remains on durvalumab and has sustained stable disease after further 28 cycles. She has not experienced any immune related adverse effects whist on durvalumab and treatment will continue until disease progression.
Case discussion
The optimal treatment duration for immunotherapy in NSCLC has not been established. Treatment until progression or unacceptable toxicity has been the standard approach in most nivolumab and atezolizumab trials (5,27,28,33). A 2-year treatment period was set in first and second-line pembrolizumab trials however has not been compared to continuous dosing due the low number of patients reaching 2 years of uninterrupted therapy.
A continuous versus fixed treatment duration has been investigated within the phase III/IV CheckMate 153 study (NCT02066636) as part of a pre-specified exploratory analysis. In this study, 220 pre-treated NSCLC patients who remained on nivolumab at 1 year were randomised (1:1) to continuous nivolumab or termination of nivolumab regardless of response status. Whilst continuous dosing was generally well tolerated, a higher rate of treatment related AEs were noted compared to the 1-year treatment arm (any grade, 39% vs. 25%; G3–4, 8% vs. 4%, respectively). Median PFS was significantly improved in the continuous dosing arm (NR vs. 10.3 months; HR 0.43, 95% CI: 0.25–0.76). Continuous dosing appeared to improve PFS regardless of PD-L1 expression and disease response (CR/PR/SD) achieved prior to randomization. A trend toward improved OS was also demonstrated in the continuous dosing arm compared to 1-year treatment arm (1-year OS rate 88% vs. 81%) with follow-up ongoing. Notably, the primary outcome of the trial was the incidence of high grade (grades 3–4 and grade 5) treatment related selected AEs so it is difficult to draw conclusions on the potential impact on PFS and OS of discontinuing nivolumab after 1 year (34). From a practical viewpoint, if response or clinical benefit has been achieved, patients may feel reluctant to stop therapy at a certain time. Future prospective randomised studies may help clarifying optimal treatment duration.
In patients who have stopped treatment due set maximum number of cycles being reached, treatment re-challenge remain an option should progression occur. Disease response after re-challenge has been demonstrated in previous responders (35). The immunogenicity of individual tumors appears pivotal in response after re-challenge as emerging data suggests that only immunological ‘hot’ or PD-L1 expressing tumors respond to re-challenge compared to responses observed in low/no PD-L1 expression tumors in immunotherapy naïve patients (36,37).
Case 5: use of checkpoint inhibitors in PD-L1 negative and poor performance status patients
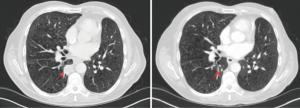
A 64-year-old male with a past medical history of type 2 diabetes mellitus, COPD and hypothyroidism was diagnosed with stage IIA (pT2bN0M0) left lung adenocarcinoma, EGFR/ALK/ROS1 wild-type and underwent a left upper lobectomy. He completed radical radiotherapy for local recurrence 12 months later. His disease relapsed again after 24 months with local recurrence invading the adjacent rib. He received one cycle of palliative chemotherapy with carboplatin/pemetrexed that was complicated by a myocardial infarction and therefore treatment was discontinued. A restaging CT scan showed stable disease the patient remained on clinical and radiological follow up for 14 months until he developed progressive lung and pleural metastasis. At that stage, he was re-challenged with carboplatin/pemetrexed but unfortunately progressed after 3 cycles of chemotherapy. He was ineligible for standard second-line immunotherapy due to performance status of 2 and PD-L1 expression (Dako 22C3) of 0%. He was enrolled on a clinical trial (NCT02733159) and received pembrolizumab 200 mg every 3 weeks. Re-staging CT after 3 cycles demonstrated partial response (Figure 3). After 8 cycles of pembrolizumab, the patient remains in a sustained partial response. No immune-related AEs have been reported.
Case discussion
Despite improved outcomes demonstrated with immunotherapy, up to 60% of patients with lung cancer receiving this treatment will not respond or yield clinical benefit. Improved patient selection and identification of predictive biomarkers remain key areas in immunotherapy research.
Firstly, unlike traditional chemotherapy, performance status may not predict tolerability and response to immunotherapy as demonstrated by the case above. Tolerability of immunotherapy appears comparable in patients >70 years with ECOG 2 compared to the overall population (38). However, retrospective analysis indicates that progression free survival is still lower in ECOG 2 patients treated with immunotherapy compared to fitter patients (39).
In terms of biomarker identification, tumour PD-L1 expression remains the most widely studied. PD-L1 expression is thought to enrich for a population who derive the greatest clinical benefit from anti-PD-1/PD-L1 therapy. However, there is variation in detection of PD-L1 with five available assays developed for PD-L1 detection in NSCLC. A prospective assessment of these 5 assays demonstrates comparable PD-L1 detection the three assays (28-8, 22C3 and SP263) that scored PD-L1 on tumour cell alone. Discordance was detected between pathologists scoring PD-L1 when using the SP142 clone, which evaluates PD-l1 expression in tumour and immune cells (40).
It is also well established that tumour PD-L1 expression is dynamic; varying over time and between tumor lesions. Exposure to cytotoxic agents or radiotherapy may also alter the tumour microenvironment as shown by differential PD-L1 expression observed on samples collected pre- and post- systemic anticancer treatments and/or radiotherapy (41-43). Overall, use of PD-L1 to predict response in clinical practice is imperfect due to heterogeneity of PD-L1 expression and quality of PD-L1 expression interpretation. This is highlighted by the fact that there is potential survival benefit also in patients with low or negative PD-L1 expression (44).
A number of other potential biomarkers are being investigated and may lead to better patients’ selection in the future. Potential biomarkers currently being evaluated are tumor mutational burden (TMB), mismatch repair (MMR) deficiency, neoantigen emergence, the number and location of tumor-infiltrating immune cells, PD-1 and PD-L1, IFN-y gene expression and T effector gene signature expression. None have yet been fully validated in prospective, randomized clinical trials.
Conclusions
Nivolumab, pembrolizumab and atezolizumab are currently approved for treatment of advanced NSCLC and currently widely used in clinical practice. Despite better tolerability than standard chemotherapy, these drugs have a different AEs profile to traditional cytotoxic therapy. It is crucial that physicians treating patient with immunotherapy are familiar with this kind of toxicity and that patients are educated in reporting these AEs without delay. This is the only way to make sure that AEs are recognized promptly and managed appropriately in order to maximize potential benefit from these drugs and improve patients’ outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pillai RN, Behera M, Owonikoko TK, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer 2018;124:271-7. [Crossref] [PubMed]
- Sgambato A, Casaluce F, Sacco PC, et al. Anti PD-1 and PDL-1 Immunotherapy in the Treatment of Advanced Non- Small Cell Lung Cancer (NSCLC): A Review on Toxicity Profile and its Management. Curr Drug Saf 2016;11:62-8. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non–Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Peters S, Gettinger S, Johnson ML, et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1–Selected Advanced Non–Small-Cell Lung Cancer (BIRCH). J Clin Oncol 2017;35:2781-9. [Crossref] [PubMed]
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. [Crossref] [PubMed]
- Haanen JB, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119-42. [Crossref] [PubMed]
- Cuzzubbo S, Javeri F, Tissier M, et al. Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur J Cancer 2017;73:1-8. [Crossref] [PubMed]
- Heinzerling L, Ott PA, Hodi FS, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer 2016;4:50. [Crossref] [PubMed]
- Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 2016;375:1749-55. [Crossref] [PubMed]
- Tay RY, Blackley E, McLean C, et al. Successful use of equine anti-thymocyte globulin (ATGAM) for fulminant myocarditis secondary to nivolumab therapy. Br J Cancer 2017;117:921-4. [Crossref] [PubMed]
- Shiuan E, Beckermann KE, Ozgun A, et al. Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy. J Immunother Cancer 2017;5:8. [Crossref] [PubMed]
- Palla AR, Kennedy D, Mosharraf H, et al. Autoimmune Hemolytic Anemia as a Complication of Nivolumab Therapy. Case Rep Oncol 2016;9:691-7. [Crossref] [PubMed]
- Helgadottir H, Kis L, Ljungman P, et al. Lethal aplastic anemia caused by dual immune checkpoint blockade in metastatic melanoma. Ann Oncol 2017;28:1672-3. [Crossref] [PubMed]
- Weber JS, Hodi FS, Wolchok JD, et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol 2017;35:785-92. [Crossref] [PubMed]
- Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012;30:2691-7. [Crossref] [PubMed]
- Weber JS, Dummer R, de Pril V, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 2013;119:1675-82. [Crossref] [PubMed]
- Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res 2015;4:560-75. [PubMed]
- Osa A, Koyama S, Uenami T, et al. P2.07-009 Monitoring Nivolumab Binding as a Method to Clarify the Residual Therapeutic Effects in Previously Treated Lung Cancer Patients. J Thorac Oncol 2017;12:S2418-9. [Crossref]
- Felix J, Lambert J, Roelens M, et al. Ipilimumab reshapes T cell memory subsets in melanoma patients with clinical response. OncoImmunology 2016;5:1136045. [Crossref] [PubMed]
- Teraoka S, Fujimoto D, Morimoto T, et al. Early Immune-Related Adverse Events and Association with Outcome in Advanced Non-Small Cell Lung Cancer Patients Treated with Nivolumab: A Prospective Cohort Study. J Thorac Oncol 2017;12:1798-805. [Crossref] [PubMed]
- Disis ML. Mechanism of action of immunotherapy. Semin Oncol 2014;41 Suppl 5:S3-13. [Crossref] [PubMed]
- Hodi FS, Kluger H, Sznol M, et al. Abstract CT001: Durable, long-term survival in previously treated patients with advanced melanoma (MEL) who received nivolumab (NIVO) monotherapy in a phase I trial. Cancer Research 2016;76:CT001. [Crossref]
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2017;377:1345-56. [Crossref] [PubMed]
- Brahmer J, Horn L, Jackman D, et al. Abstract CT077: Five-year follow-up from the CA209-003 study of nivolumab in previously treated advanced non-small cell lung cancer (NSCLC): Clinical characteristics of long-term survivors. Cancer Res 2017;77:CT077. [Crossref]
- Brahmer J, Rodríguez-Abreu D, Robinson A, et al. OA 17.06 Updated Analysis of KEYNOTE-024: Pembrolizumab vs Platinum-Based Chemotherapy for Advanced NSCLC With PD-L1 TPS ≥50%. J Thorac Oncol 2017;12:S1793-4. [Crossref]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti–Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non–Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. [Crossref] [PubMed]
- von Pawel J, Syrigos K, Mazieres J, et al. 1314P - Association Between Immune-Related Adverse Events (irAEs) and Atezolizumab Efficacy in Advanced NSCLC: Analyses From the Ph III Study OAK. ESMO 2017 Meeting, Madrid. Ann Oncol 2017;28:v460-96.
- Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 2017;28:583-9. [PubMed]
- Hasan Ali O, Diem S, Markert E, et al. Characterization of nivolumab-associated skin reactions in patients with metastatic non-small cell lung cancer. Oncoimmunology 2016;5:e1231292. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Spigel DR, McLeeod M, Huessein MA, et al. Randomized results of fixed-duration (1-yr) vs continuous nivolumab in patients (pts) with advanced non-small cell lung cancer (NSCLC). Ann Oncol 2017;28:v460-96. [Crossref]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Larkin J, Minor D, D'Angelo S, et al. Overall Survival in Patients With Advanced Melanoma Who Received Nivolumab Versus Investigator's Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J Clin Oncol 2018;36:383-90. [Crossref] [PubMed]
- Weber J, Gibney G, Kudchadkar R, et al. Phase I/II study of metastatic melanoma patients treated with nivolumab who had progressed after ipilimumab. Cancer Immunol Res 2016;4:345-53. [Crossref] [PubMed]
- Migliorino MR, Gelibter A, Grossi F, et al. 1320P - Use of nivolumab in elderly patients with advanced Non-Squamous NSCLC: results from the Italian Expanded Access Program (EAP). ESMO 2017 Meeting, Madrid. Ann Oncol 2017;28:v460-96. [Crossref]
- Taniguchi Y, Tamiya A, Isa S, et al. 1162P - Predictive factors for poor progression-free survival in patients with non-small-cell lung cancer treated with nivolumab. ESMO 2017 Meeting, Madrid. Ann Oncol 2017;28:v403-27.
- Tsao MS, Kerr KM, Yatabe Y, et al. PL 03.03 Blueprint 2: PD-L1 Immunohistochemistry Comparability Study in Real-Life, Clinical Samples. WCLC IASCL Meeting, Yokohama, 2017.
- Mukherji D, Jabbour MN, Saroufim M, et al. Programmed Death-Ligand 1 Expression in Muscle-Invasive Bladder Cancer Cystectomy Specimens and Lymph Node Metastasis: A Reliable Treatment Selection Biomarker? Clin Genitourin Cancer 2016;14:183-7. [Crossref] [PubMed]
- Wimberly H, Brown JR, Schalper K, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in Breast cancer. Cancer Immunol Res 2015;3:326-32. [Crossref] [PubMed]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. [Crossref] [PubMed]
- Passiglia F, Bronte G, Bazan V, et al. PD-L1 expression as predictive biomarker in patients with NSCLC: a pooled analysis. Oncotarget 2016;7:19738-47. [Crossref] [PubMed]


