Use of efficient purse-string stapling technique for esophagogastric anastomosis in minimally invasive Ivor Lewis esophagectomy
Introduction
Total minimally invasive esophagectomy (MIE), performed with neck or chest anastomosis, comprises three surgical approaches: laparoscopic transhiatal, laparoscopic-thoracoscopic McKeown type 3-incision and laparoscopic-thoracoscopic Ivor-Lewis. In minimally invasive Ivor-Lewis esophagectomy, intrathoracic esophagogastric anastomosis can be accomplished by both handsewn and stapled (circular or side-to-side) technique (1,2). Investigators have demonstrated an obvious priority of the stapler technique (3), nevertheless, intrathoracic side-to-side stapled anastomosis is yet applied in limited number of cases. By using a circular-stapling device, there are two steps in intrathoracic anastomosis: first, placement of the anvil into the proximal esophagus; second, alignment and docking of the anvil to the shaft of stapler to achieve anastomosis. Although more surgeons adopt circular-stapled anastomosis procedure (4), it is still technically demanding, especially for the appropriate placement of the anvil into the esophageal remnant. EST is a new and easier method of placing the anvil under thoracoscopic monitoring.
Between August 2012 and April 2013, 14 patients (10 males and 4 females) accepted planned laparoscopic-thoracoscopic Ivor-Lewis esophagectomy. The median age of the patients was 60 (range, 45-77) years. All the patients had resectable (cT1-3, N0-1, M0), histologically proven esophageal squamous cell carcinoma. The intrathoracic anastomosis was performed with EST after obtaining informed consent from the patients. The operations were finished without conversion to open. In all the cases, anastomsis were performed above the level of azygos vein arch with intact anastomotic doughnuts. All the patients recovered uneventfully after surgery. After receiving esophageal contrast barium swallow to exclude anastomotic leak, all the patients were allowed to have clear liquids on the 6th postoperative day and semiliquid diet on the 7th postoperative day. Their pathological staging varied from stage I to stage IIIA, 64% of them (9/14) were referred to receive adjuvant radiotherapy after surgery.
Technique
General anesthesia is induced and intubation is achieved via a double-lumen endotracheal tube. Laparoscopic gastric mobilization is done as previously described (5). Creation of the gastric conduit is performed by using three firings of a linear stapler (Echelon 60, Ethicon Endo-Surgery) without complete separation. Under laparoscopic monitoring, resection margin of the gastric conduit is reinforced by running suture and a feeding jejunal tube is placed.
During the thoracoscopic phase, the patient is placed in left lateral decubitus position with arms extended to 90° and elbows flexed to 90°. The surgeon stands on ventrally part of the patient. Four ports are made: a 1 cm optical port is placed in the 7th intercostal space at mid-axillary line; the utility port, a 4 cm incision expanded with a protection sleeve, is placed in the 4th intercostal space at the anterior axillary line; the other two ports are 1.5 cm incisions placed in the anterior axillary line at the 6th intercostal space and scapular line at the 7th intercostal space, respectively. Then, the mediastinal pleura is opened, and the arch of the azygos vein is mobilized and transected. Thoracic esophagus is mobilized until a safe oncological distance from the tumor is ensured. Thereafter, the esophagus-surrounded mediastinal lymphatic tissue, subcarinal lymph nodes and the recurrent laryngeal nerve lymph nodes are retrieved. The gastric conduit is delivered into the thoracic cavity by transhiatal gastric pull-up.
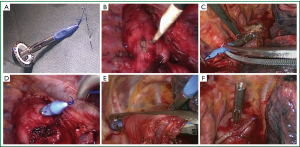
Before anastomosis, an anvil set is established: first, a 2-0 Prolene suture (Ethicon, Somerville, N.J.) is secured at the tip of a trocar included in the circular-stapling device (ECS 25, Ethicon Endo-Surgery) with a knot tied over ten times. Then, the central rod of the anvil is connected with the trocar to create the anvil set. A 3 cm incision is made to cut through the esophageal wall, 3-5 cm proximal to the tumor margin. The anvil set is introduced into the thorax through the utility port and subsequently into the esophageal cavity through the esophageal incision. The Prolene suture accompanying the trocar pierces the esophageal wall 2-3 cm above the esophageal incision. The suture and the anvil rod are pulled out from the esophageal wall before the esophagus is transected near where the central rod is exposed by a linear-stapling device (Echelon 60, Ethicon Endo-Surgery). The suture is pulled again to ensure the whole body of the central rod is exposed and the anvil is clung to the esophagus (Figure 1A-F). After detaching the trocar from the anvil rod, the cardia is transected so that the esophageal specimen is retrieved for freeze pathological examination of the margin. The shaft of the circular stapler is introduced through the utility port and then inserted into the gastric conduit through the cardiac incision. After the circular stapler is docked with the anvil, the esophagogastric anastomosis is completed in an end-to-side fashion. The gastric conduit is finally completed with additional firings of linear stapler. The anastomosis is inspected, if there is any uncertainty of the anastomosis, the esophageal stump horns are embedded with mattress-suture, an omental flap is wrapped around the anastomosis and a mediastinal drainage tube is placed (Video 1).
Comments
As minimally invasive Ivor-Lewis esophagectomy is increasingly performed, there is growing concern about the complexity of making an intrathoracic esophagogastric anastomosis. Since 2011, we have performed over 100 cases of MIE with intrathoracic anastomosis. In our cases, transoral (Orvil device) and transthoracic (handsewn purse-string stapled anastomosis) technique (6) has been used in approximately equivalent numbers of cases. Transoral introduction of anvil is performed by an anesthesiologist using an orogastric tube attached with a prepared pretilted anvil’s head. A small exit hole is made on the esophageal stump to enable the gastric tube to advance through until the anvil reaches the right position. However, making the esophageal stump hole is difficult, undersized hole impedes the passing through of gastric tube and oversized hole induces dislocation of the anvil. Alternatively, surgeons can perform handsewn purse-string stapled anastomosis without the help of an anesthetist; nevertheless, it is more technically challenging for both fixing the anvil and transecting the esophagus with proper mucosa remained.
EST was originally applied in the gastrojejunostomy in laparoscopic distal gastrectomy with excellent results (7). In order to search new options for intrathoracic anastomosis in endoscopic Ivor-Lewis esophagectomy, we perform gastroesophageal anastomosis with EST. Comparing with the transoral technique, EST causes less damnification of the proximal esophageal mucosa and less spillage of saliva in the wound by avoiding an introduction tube with pretilted anvil from mouth into thorax. Unlike the handsewn purse-string technique, in the EST procedure, the esophagus is transected by a linear stapler after the anvil rod penetrates the esophageal wall. Stabilization of the esophagus by the stapler facilitates passage of the anvil rod, besides; stapler transection allows proper mucosa remaining in esophageal stump. In both transoral and handsewn purse-string stapled procedures, incomplete circumferential anastomotic margin or even anastomotic leakage may occur if the exit hole of the anvil rod is excessively or eccentrically extended during anastomosis. To prevent this complication, additionally purse-string sutures around the anvil rod are often recommended (8). By utilizing EST, the troca with its fixed knot on the anvil set facilitates the passage of the rod through a minimal exit hole when being pulling out. Hence, the risk of extension of the exit hole is minimized and additional suture is spared.
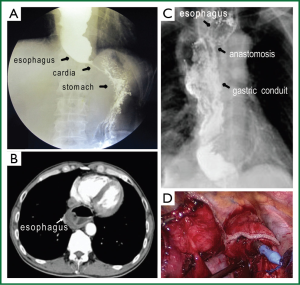
By using handsewn purse-string stapled technique, the whole circle of esophageal wall is collected around the anvil rod to be involved in the anastomosis. As a result of mechanical obstruction, patients with advanced esophageal cancer often have expanded remnant esophagus, besides, the esophageal muscular layer are thickened in some cases. Under these circumstances, excessive tissue involvement in the anastomotic doughnuts will impede the anastomosis. When using EST, usually only a portion of the resection margin of the esophageal stump is involved in anastomosis, proper amount of tissue involvement facilitates the anastomsis. Actually, in this case series, we encountered a patient with significant mega-esophagus, which required two firings of a 6 cm linear-stapling device to complete the esophageal stump. For this patient, making a handsewn purse-string stapled anastomosis is obviously difficulty, so EST was perfored with satisfactory result (Figure 2A-D).
In this study, anvil fixation time (from incising the esophageal wall to esophageal transection) is about 8 minutes (range, 6-15 minutes), less than the time for fixing the anvil with handsewn purse-string stapled technique (10 min; range, 8-22 min). Because endoscopic esophagogastric anastomosis with EST requires less handmade cutting and suture, it is less technically demanding and easier to be employed by surgeons who are initiating their experience with endoscopic Ivor-Lewis esophagectomy. Remarkably, as the anvil set require longer intact thoracic esophagus to be inserted in, EST is especially appropriate for the patients with distal esophageal cancer or dilated esophageal cavity.
In summary, the application of EST facilitates circular-stapled intrathoracic gastro-esophageal anastomosis. The technique is easy to handle and is an attractive option for surgeons when performing minimally invasive Ivor-Lewis esophagectomy.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Watson DI, Davies N, Jamieson GG. Totally endoscopic Ivor Lewis esophagectomy. Surg Endosc 1999;13:293-7. [PubMed]
- Cadière GB, Dapri G, Himpens J, et al. Ivor Lewis esophagectomy with manual esogastric anastomosis by thoracoscopy in prone position and laparoscopy. Surg Endosc 2010;24:1482-5. [PubMed]
- Blackmon SH, Correa AM, Wynn B, et al. Propensity-matched analysis of three techniques for intrathoracic esophagogastric anastomosis. Ann Thorac Surg 2007;83:1805-13; discussion 1813.
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [PubMed]
- Pennathur A, Awais O, Luketich JD. Technique of minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg 2010;89:S2159-62. [PubMed]
- Zhang RQ, Xia WL, Kang NN, et al. Pursestring stapled anastomotic technique for minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg 2012;94:2133-5. [PubMed]
- Omori T, Oyama T, Akamatsu H, et al. A simple and safe method for gastrojejunostomy in laparoscopic distal gastrectomy using the hemidouble-stapling technique: efficient purse-string stapling technique. Dig Surg 2009;26:441-5. [PubMed]
- Murr MM, Gallagher SF. Technical considerations for transabdominal loading of the circular stapler in laparoscopic Roux-en-Y gastric bypass. Am J Surg 2003;185:585-8. [PubMed]


