Acute liver failure with extreme hyperbilirubinemia secondary to endocarditis-related severe mitral and tricuspid regurgitation: a challenge and an opportunity for surgeons
History of presenting illness
A 43-year-old man with unremarkable past health presented to the medical unit for pyrexia of unknown origin. Physical exam noted a pan-systolic murmur and bilateral lower limbs rash. He had no recent history of dental or surgical procedures. Blood culture grew streptococcus sanguinis on repeated samples. Transesophageal echocardiogram (TEE) revealed multiple vegetations over his anterior mitral valve leaflet. The largest vegetation had a diameter of around 1 cm. In addition, there was a perforation over the A3 scallop causing severe eccentric mitral regurgitation (MR). The effective regurgitant orifice (ERO) area was 0.5 cm2 and his left ventricular ejection fraction (LVEF) was 68%. He was referred to the cardiothoracic unit by the cardiologists and surgery was recommended. He strongly refused surgical intervention for his infective endocarditis and opted for conservative treatment. He was treated with intravenous penicillin G plus gentamicin for 4 weeks. He remained stable on conservative treatment with only mild shortness of breath on exertion. His follow-up echocardiogram 2 months after discharge showed dilated left ventricle with left ventricular end-diastolic dimension of 6.7 cm and impaired LVEF of 45%. There was a flail anterior mitral valve leaflet due to ruptured chordae at the A3 scallop causing severe eccentric MR (ERO was 0.94 cm2 with regurgitant volume of 98 mL). Moderate tricuspid regurgitation with right ventricular systolic pressure of 52 mmHg was also noted. New onset atrial fibrillation was diagnosed during follow up and anticoagulation with warfarin was started. Coronary angiogram was normal. Eventually, the patient agreed to elective surgical repair of his leaking mitral and tricuspid valves.
Preoperative condition and management
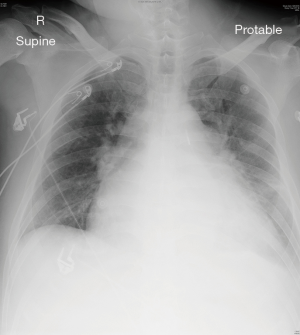
Ten days before the scheduled operation, he was admitted through the emergency department due to sudden worsened heart failure with bilateral leg edema. Blood tests showed impaired renal and liver function. Upon admission, total bilirubin level was 200 µmol/L, the creatinine level was 158 µmol/L, and the international normalized ratio (INR) was 8. Chest radiograph showed cardiomegaly and features of acute pulmonary edema (Figure 1). The patient required high dose oxygen but did not require intubation initially. However, he soon required moderate dose of noradrenaline, regular furosemide injections, and intra-aortic balloon pumping (IABP) to achieve afterload reduction for his heart failure secondary to severe MR.
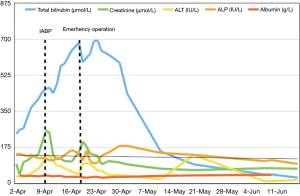
Hepatitis work-up showed negative results and no hepatotoxic medications had been given. Ultrasound of the abdomen revealed congested liver with no evidence of cholestasis. After commencement of inotropes and IABP support, the patient’s renal function, alanine transaminase (ALT), and albumin levels were normalized, but his total bilirubin level continued to rise (Figure 2). The highest bilirubin level reached 700 µmol/L. The patient stayed at the high dependency unit and intensive care unit (ICU) for a total of 9 days before operation. Emergency operation was then arranged despite the climbing hyperbilirubinemia, in view of his deteriorating hemodynamics and persistent liver congestion even with maximal medical therapy.
Intraoperative findings and postoperative care
Emergency valve surgery was performed via a median sternotomy with the use of cardiopulmonary bypass. After induction of anesthesia, central venous pressure (CVP) was 36 mmHg associated with severe tricuspid regurgitation. The tricuspid annular diameter was measured at 4.29 cm by TEE. Intraoperative findings were consistent with history of infective endocarditis with old calcified vegetations and ruptured chordae at A2 and A3. The calcified vegetation was resected and sent for microbiological investigation. The A3 perforation was plicated. Mitral and tricuspid annuloplasty were performed using a size-32 Memo 3D ReChord ring (LivaNova, London, UK) and a size-30 tricuspid Physio ring (Edwards Lifesciences, Irvine, CA, USA), respectively. Two artificial cords (CV4 Gore-Tex suture, Gore Medical, Flagstaff, AZ, USA) were implanted and attached to A2 and A3 sites, following the ReChord guidance as descripted previously (1). Postoperative TEE showed no residual MR and mild tricuspid regurgitation. His CVP was reduced to 15 mmHg after weaning from cardiopulmonary bypass. As the patient still showed signs of right heart failure, IABP support was maintained and nitric oxide was given through the endotracheal tube. Levosimendan was started with a 12 µg/kg loading dose over 10 minutes followed by 0.1 µg/kg/min infusion for 24 hours.
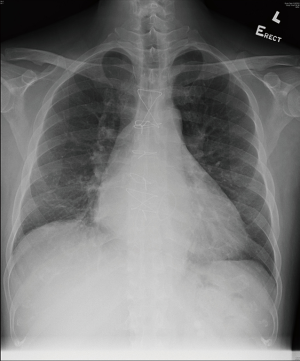
The patient was able to wean off nitric oxide on postoperative day 1 and was extubated 38 hours after the operation. He stayed in the ICU for a total of 2 days and the IABP was removed 4 days after surgery. Antibiotic (amoxicillin and clavulanic acid) was stopped following the confirmation of negative bacterial culture from his resected valve vegetation. His postoperative recovery was otherwise uneventful and he was discharged 12 days after his operation. His total bilirubin level gradually normalized 2 months after the operation. His chest X-ray also showed reduced heart size (Figure 3). He was in excellent condition upon his postoperative 18-month follow-up whereas his echocardiogram showed trivial mitral and tricuspid regurgitation only, with a LVEF of 58%. His CHA2DS2-VASc score was 1 and he was put on aspirin 80 mg daily for stroke prophylaxis.
Discussion
Acute infective endocarditis with severe MR is usually indication for early surgery. In our case, the patient refused surgery initially despite strong recommendations. He presented with decompensated heart failure with multi-organ dysfunction before finally undergoing emergency mitral and tricuspid valve repair. Although hyperbilirubinemia is a known risk factor for open heart operations (2-5), emergency surgical intervention was then the only effective option of survival for this young patient. As far as therapeutic approach is concerned, whenever feasible valve repair is always preferred over replacement with either a tissue or mechanical valve prosthesis as repair provides better long-term survival with improved quality of life, especially for young patients. Meanwhile, the safety of catheter-based intervention such as the application of MitraClip in infective endocarditis-related mitral and tricuspid regurgitation is uncertain, not to mention that the long-term durability and effectiveness of the MitraClip intervention in the current setting has never been proven.
Acute right heart failure can cause liver dysfunction. In a study on ICU patients with acute liver failure, about 6% were related to congestive heart failure (6). Deranged liver function tests are commonly seen in patients with acute heart failure. Among those patients with acute heart failure, 46% had abnormal aspartate aminotransferase alone, 31% had abnormal ALT alone, 33% had high bilirubin alone, and 44% had albumin derangements alone. Only 29% of the patients had all liver function parameters within the normal ranges (4).
Pathophysiology
The pathophysiology of liver failure in heart failure is incompletely understood (7). The liver has a dual blood supply (i.e., hepatic artery and portal vein), making it less prone to hypoperfusion. Studies on the pathogenesis of ischemic hepatitis stated that systemic hypotension or shock alone does not lead to ischemic hepatitis. The vast majority of patients with ischemic hepatitis had severe underlying cardiac disease that led to congestion of the liver (8). Therefore, two components are required to induce liver injury, which include hepatic congestion from venous hypertension and decreased oxygen delivery from decreased cardiac output. Once oxygen delivery is reduced to a critical level, hepatic hypoxia initiates a process that results in hemorrhagic centrilobular necrosis. Venous hypertension results in periventricular sinusoidal congestion, endothelial injury, replacement of hepatocytes with erythrocytes, and ultimately, centrilobular necrosis (9,10). Congestive heart failure induced acute liver failure patient has been shown to have elevated CVP and reversal of portal vein flow in support of this theory (6).
Predictive value of liver functions in acute heart failure
The studies on the role of abnormal liver function test in the prediction of clinical outcome were somewhat contradicting. Shinagawa et al. (11) studied clinical implications of liver dysfunction in acute heart failure, and found that elevated total bilirubin on admission was a marker of poor prognosis. Ambrosy et al. (5) identified high bilirubin and low albumin, but not elevated ALT, was associated with poor outcomes in acute heart failure. However, Biegus et al. (4) demonstrated that patients with markedly elevated transaminases as well as those with low albumin concentration but not bilirubin had significantly higher mortality at 6 months.
The inconclusive results may be due to the following reasons. Liver function test is affected by multiple confounding factors. Albumin can be low due to hemodilution, aging, poor nutrition etc. which can also contribute to poor prognosis. The cause of bilirubin elevation can be different in acute decompensated congestive heart failure and de novo heart failure (4). These studies included a heterogenous group of cardiac disease with differing chronicity, which may have different underlying pathophysiology. Moreover, the liver function test may indirectly reflect cardiac function. Liver congestion in the setting of heart failure suggests some degree of right heart dysfunction. This was supported by hemodynamic studies showing that patients with acute liver failure also had elevated right atrial pressure (6). However, right heart function, an important prognostic factor, was not properly assessed in most of the studies. Therefore, baseline results may not be useful in predicting outcome but a deteriorating trend may indicate failing medical treatment. As in our patient, the sole increase of bilirubin despite inotropic support and IABP suggested he was deteriorating clinically.
There are limited studies showing the relationship of acute liver failure and cardiac surgery outcomes. Most of the studies used Child Pugh score and model for end-stage liver disease (MELD) score, which are indices for patients with chronic liver disease.
Levosimendan for acute right heart failure
Our patient had biventricular failure due to decompensation from severe mitral and tricuspid regurgitation. After valve repair and weaning off cardiopulmonary bypass, the left ventricle contractility was good without hypokinesia shown on TEE. However, the right ventricle was dilated and contraction was sluggish which led to a low cardiac output state despite postoperative IABP support. Levosimendan was given to improve hemodynamics. Levosimendan is a calcium sensitiser and an inodilator used in acute decompensated heart failure. The pharmacologic effects of levosimendan include increasing cardiac contractility mediated by calcium sensitization of troponin C, vasodilation through the opening of potassium channels on the sarcolemma of smooth muscle cells in the vasculature and cardioprotection through the opening of mitochondrial potassium channels in the cardiomyocytes (12-14). It has been shown to improve right ventricular function by reducing the right ventricular afterload, improving right ventricular contractility and improving diastolic function of the right ventricle. It was effective in our patient, allowing him to wean off inotropic and IABP support after 24-hour infusion of levosimendan.
Conclusions
Severe mitral and tricuspid regurgitation may cause hyperbilirubinemia. As in our case, prompt surgery is the sole definitive treatment for this type of acute cardiogenic liver failure despite high blood levels of bilirubin being a relative contraindication to open heart surgery. The management of this group of patients with critical biventricular heart failure remains challenging and thus the choice of therapeutic approach should be individualized.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wan S, Lee AP, Attaran S, et al. Mitral valve repair using a semirigid ring: patient selection and early outcomes. Asian Cardiovasc Thorac Ann 2016;24:647-52. [Crossref] [PubMed]
- Bøhmer T, Kjekshus E, Nitter-Hauge S. Studies on the elimination of bilirubin pre-operatively in patients with mitral valve disease. Eur Heart J 1994;15:10-6. [Crossref] [PubMed]
- Suman A, Barnes DS, Zein NN, et al. Predicting outcome after cardiac surgery in patients with cirrhosis: a comparison of Child-Pugh and MELD scores. Clin Gastroenterol Hepatol 2004;2:719-23. [Crossref] [PubMed]
- Biegus J, Zymliński R, Sokolski M, et al. Liver function tests in patients with acute heart failure. Pol Arch Med Wewn 2012;122:471-9. [PubMed]
- Ambrosy AP, Vaduganathan M, Huffman MD, et al. Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST trial. Eur J Heart Fail 2012;14:302-11. [Crossref] [PubMed]
- Saner FH, Heuer M, Meyer M, et al. When the heart kills the liver: acute liver failure in congestive heart failure. Eur J Med Res 2009;14:541-6. [PubMed]
- Lopez-Delgado JC, Esteve F, Javierre C, et al. Short-term independent mortality risk factors in patients with cirrhosis undergoing cardiac surgery. Interact Cardiovasc Thorac Surg 2013;16:332-8. [Crossref] [PubMed]
- Seeto RK, Fenn B, Rockey DC. Ischemic hepatitis: clinical presentation and pathogenesis. Am J Med 2000;109:109-13. [Crossref] [PubMed]
- Kanel GC, Ucci AA, Kaplan MM, et al. A distinctive perivenular hepatic lesion associated with heart failure. Am J Clin Pathol 1980;73:235-9. [Crossref] [PubMed]
- Arcidi JM Jr, Moore GW, Hutchins GM. Hepatic morphology in cardiac dysfunction: a clinicopathologic study of 1000 subjects at autopsy. Am J Pathol 1981;104:159-66. [PubMed]
- Shinagawa H, Inomata T, Koitabashi T, et al. Prognostic significance of increased serum bilirubin levels coincident with cardiac decompensation in chronic heart failure. Circ J 2008;72:364-9. [Crossref] [PubMed]
- Pollesello P, Papp Z. The cardioprotective effects of levosimendan: preclinical and clinical evidence. J Cardiovasc Pharmacol 2007;50:257-63. [Crossref] [PubMed]
- Sorsa T, Pollesello P, Solaro RJ. The contractile apparatus as a target for drugs against heart failure: interaction of levosimendan, a calcium sensitiser, with cardiac troponin C. Mol Cell Biochem 2004;266:87-107. [Crossref] [PubMed]
- Pataricza J, Hõhn J, Petri A, et al. Comparison of the vasorelaxing effect of cromakalim and the new inodilator, levosimendan, in human isolated portal vein. J Pharm Pharmacol 2000;52:213-7. [Crossref] [PubMed]