Immunotherapy in previously treated non-small cell lung cancer (NSCLC)
Introduction
Cancer of the lung and bronchus continues to be the leading cause of deaths attributed to cancer in the United States, with an estimated 155,870 deaths from lung cancer in 2017 (1). Unfortunately, the majority are diagnosed at an advanced disease stage at the time of presentation which portends a poor prognosis with 5-year survival rates of approximately 4% (2).
Overall, meaningful advances in improving outcomes in non-small cell lung cancer (NSCLC) have been scarce in this past decade. Notably, the outcomes are more favorable for the subset of patients with actionable mutations that are responsive to appropriate targeted treatments. The reality is that for the majority of patients, whose tumor does not harbor an actionable mutation, the median survival is approximately 12 months (3). Standard cytotoxic chemotherapies in the second-line setting following platinum-based chemotherapy for metastatic NSCLC generate low objective response rate (ORR), and marginal survival benefit (4,5).
Chemotherapy combinations used in the salvage therapy setting have not resulted in improved survival. However, the combination of docetaxel with ramucirumab, an anti-vascular endothelial growth factor receptor 2 monoclonal antibody, demonstrated superiority in overall survival relative to docetaxel alone and is available as a combination strategy for salvage therapy (6).
The development of immune checkpoint inhibitors, targeting the programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway, has changed how we treat NSCLC, making this an established therapeutic strategy for individuals with advanced disease. Cancer survival, particularly for advanced stage NSCLC, is expected to increase in light of recent advances in immunotherapy (7).
PD1/PD-L1 pathway
Oncogenesis is a multifaceted, multistep process characterized by the accumulation of genetic and epigenetic alterations that drive or reflect tumor progression. These alterations set apart cancer cells from normal cells, triggering the immune system to recognize these as foreign. However, tumors are rarely fully rejected by the immune system, reflecting that cancer cells have the ability to maintain an immunosuppressive microenvironment (8).
PD-1 is a receptor found on T cells that are activated, and functions as a counterbalancing force for T-cell receptor (TCR) signaling and acts as an “off switch” for the immune system to prevent autoimmunity (9). The PD-1 receptor functions mainly at the site of peripheral tissues, allowing T cells to interact with the PD-1 ligands [PD-L1 (B7-H1) and PD-L2 (B7-DC)] (9,10). The upregulation of PD-L1 observed in the setting of malignancy, downregulates primed T cells, which leads to cancer evasion, escape and progression (11,12).
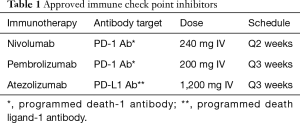
In summary, the immune check point pathway plays an important role in dampening immunosurveillance for tumors, leading to tumor immune evasion, and is therefore an attractive therapeutic target. Currently, there are three immune check point inhibitors approved in the salvage therapy setting: nivolumab, pembrolizumab and atezolizumab (see Table 1). Pembrolizumab has been recently indicated for treatment-naïve patients with advanced NSCLC with tumor proportion score (TPS) ≥50% or high tumor PD-L1 expression (13), and in combination with platinum and pemetrexed for metastatic NSCLC with non-squamous histology (14).
Full table
Immunotherapy agents in clinical practice (seeTable 2)
Full table
Nivolumab
Nivolumab is a fully human IgG4 anti-PD-1 monoclonal antibody and serves to restore antitumor immunity (15,16). Nivolumab was the first immune check point inhibitor in this class to receive approval for pre-treated advanced NSCLC following platinum-doublet chemotherapy.
Nivolumab was reported to have activity with manageable side effects in patients with multiple solid tumors, including NSCLC (17). This phase Ib study included a cohort of 122 patients with metastatic NSCLC, with more than half of the patients (55%) having received three or more prior treatments. Nivolumab was investigated in differing dosing regimens: 1, 3, or 10 mg/kg of nivolumab every 2 weeks, for maximum of 2 years. The ORR was 18%, with durable responses and duration of response of 17 months. It is noteworthy that the responses were seen in the three dosing regimens and were also seen independent of histology. The prolonged duration of response to nivolumab is in stark contrast to that with chemotherapy where responses are often short-lived. The remarkable activity seen with an immune checkpoint inhibitor in a heavily pre-treated cohort of patients with NSCLC generated enthusiasm for further investigation of this class of agents. However, it was clear that the clinical benefit was limited to a subset of patients, thus calling for identification of predictive biomarkers for patient selection. Serious treatment-related adverse events (AEs) was reported in 14% of cases. Among AEs with immune-related etiology, pneumonitis, colitis, hepatitis, and thyroiditis were reported. Immune-mediated pneumonitis was described in 3% and serious (grade 3/4) pneumonitis was reported in 1% of cases. Of note, three deaths were reported due to immune-mediated pneumonitis (two in NSCLC and one in colon cancer patients). Management of early pneumonitis required treatment discontinuation, initiation of glucocorticoids or both with successful outcomes. In three patients with severe pneumonitis, additional immunosuppression was required beyond glucocorticoids and infliximab, mycophenolate, or both were administered.
In the NSCLC cohort, nivolumab at 3 mg/kg was associated with encouraging survival outcomes with a 2-year OS rate of 42% (18). The median OS was 14.9 months in 37 patients. No association was reported between PD-L1 expression and outcomes. Among 17% of patients who achieved objective responses, these responses were durable, with median duration of response of 17 months. Responses were seen independent of histology (squamous versus nonsquamous). The rate of serious drug-related AEs was 14%. Three treatment-related deaths due to pneumonitis occurred (18). The long-term survival data of this phase Ib study revealed the 5-year OS to be 16%, notable given that the 5-year OS historically has been less than 5% (19). Nivolumab also demonstrated activity in the phase II trial setting for patients with metastatic NSCLC of squamous histology with ORR of 15% and 1-year OS of 42% (CheckMate 063) in the third-line setting and beyond (20).
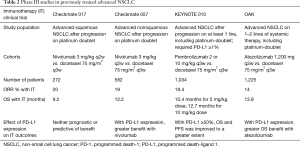
These data prompted the conduct of two large phase III trials, CheckMate 017 and CheckMate 057, investigating nivolumab in patients with pre-treated advanced NSCLC (21,22).
CheckMate 017 (21) sought to compare the safety and efficacy of nivolumab to docetaxel in patients with progressive advanced squamous cell NSCLC following treatment with platinum-based doublet chemotherapy. A total of 272 patients were randomized to nivolumab at 3 mg/kg IV every 2 weeks (n=135 patients) or docetaxel at 75 mg/m2 IV every 3 weeks (n=137 patients). The primary endpoint was OS. Secondary endpoints included efficacy according to tumor PD-L1 expression. Most patients in both arms were current or former smokers. The primary endpoint of this study was met with 1-year OS of 42% in the nivolumab arm versus 24% in the docetaxel arm, and higher ORR with nivolumab compared to docetaxel (20% vs. 9%). The durability of response was longer with nivolumab treatment compared to docetaxel, with median duration of response not reached in the nivolumab arm compared with 8.4 months in the docetaxel arm. The benefit achieved with nivolumab was independent of PD-L1 expression. Serious AEs were lower in frequency in the nivolumab arm (7%) versus docetaxel (55%). No treatment-related deaths were reported in the nivolumab arm; whereas there were three deaths attributed to docetaxel (one patient died due to interstitial lung disease, one patient died due to pulmonary hemorrhage, and one died due to complications of sepsis). CheckMate 017 supported the approval of nivolumab therapy for advanced squamous NSCLC in previously treated patients following platinum-doublet chemotherapy in March of 2015.
CheckMate 057 investigated the safety and efficacy of nivolumab compared to docetaxel in patients with progressive advanced non-squamous NSCLC following treatment with platinum-based doublet chemotherapy (22). A total of 582 patients were randomized to nivolumab at 3 mg/kg IV every 2 weeks (n=292 patients) or docetaxel at 75 mg/m2 IV every 3 weeks (n=290 patients). The primary endpoint was OS. Secondary endpoints included efficacy according to tumor PD-L1 expression. Most patients in both arms were current or former smokers. The primary endpoint of this study was met with 1-year OS of 51% in the nivolumab arm versus 39% in the docetaxel arm. The median OS in patients receiving nivolumab was 12.2 as compared to 9.4 months in the docetaxel arm. The rate of ORR was superior with nivolumab (19%) as compared to docetaxel (12%) in the overall study population. Similarly to what had been previously reported, response duration was longer with nivolumab (17.2 months) versus docetaxel (5.6 months). Interestingly, treatment with nivolumab did not result in longer progression-free survival (PFS) as compared to docetaxel; notable for crossing of the PFS curves. Although this study met its primary endpoint, the benefit was greater across all the efficacy end points among patients with tumor PD-L1 expression compared to those with negative PD-L1 expression. A pre-specified subgroup analysis suggested that patients with negative PD-L1 in the nivolumab arm had comparable OS to those in the docetaxel arm. However, given the favorable tolerability and durability of responses, nivolumab is considered a reasonable treatment option in this subgroup. Serious drug-related AEs were lower and less common with nivolumab as compared to docetaxel. One patient died in each of the two groups. One patient in the nivolumab arm died due to encephalitis attributed to treatment, and one patient in the docetaxel arm died due to treatment-related febrile neutropenia. These results supported the use of nivolumab for pre-treated advanced nonsquamous NSCLC following platinum-doublet chemotherapy in October of 2015.
In CheckMate 017, with 2-year follow-up, nivolumab continued to show a significant benefit with regards to OS and demonstrated significantly greater median duration of response, notable for a response duration of 25.2 months in the nivolumab arm versus 8.4 months in the docetaxel arm. Similarly in CheckMate 057, OS superiority was maintained in the nivolumab arm. The median duration of response was also significantly longer in the nivolumab arm, notable for a response duration of 17.2 months in the nivolumab arm versus 5.6 months in the docetaxel arm.
In summary, with ≥2 y follow-up from these two landmark trials (CheckMate 017 and 057), the benefits of nivolumab persisted with OS benefit and significantly improved rates of durable responses as compared to docetaxel (23). Based on the strength of these results, nivolumab is currently deemed to be an effective treatment used in the routine care of patients with advanced NSCLC in the salvage setting.
Pembrolizumab
Pembrolizumab is a humanized IgG4 kappa isotype anti-PD-1 monoclonal antibody. The end result disrupts the inhibitory signals in T cells allowing better tumor recognition by cytotoxic T cells and improved tumor cell death.
KEYNOTE-001 was a phase I study that included patients with metastatic NSCLC in multiple expansion cohorts. Patients with treatment-naïve and pre-treated NSCLC received pembrolizumab in the following dose finding cohorts: 2 or 10 mg/kg IV every 3 weeks (6,24). Primary endpoints were safety, tolerability, and efficacy of pembrolizumab. ORR was 19.4% in the overall population, and ORR of 18.0% in pre-treated patients (n=394) and 24.8% in the treatment naïve patients (n=101). These responses were durable with a median response duration of 12.5 months. The analysis of PD-L1 was used as a potential predictive biomarker. A proportion score of ≥50% was associated with a superior ORR, longer PFS and OS as compared to <50% in both pre-treated and untreated patients. This suggested that tumors with high PD-L1 may be more responsive to immune check point inhibitors. The most common AEs were fatigue, anorexia, and pruritus. Serious AEs were reported in 9.5% of patients. Immune-related adverse events (IRAE) reported >2% of patients included infusion-related reactions (3%), hypothyroidism (6.9%), and pneumonitis (3.6%). Serious pneumonitis was uncommon (1.8%), with 1 patient death (0.2%). Given the greater degree of benefit in patients with tumor PD-L1 ≥50%, pembrolizumab 2 mg/kg every 3 weeks was approved for pre-treated advanced NSCLC following platinum-doublet chemotherapy.
In an updated report on the survival of patients with advanced NSCLC on KEYNOTE-001, the median OS was 22.1 months for treatment-naive patients and 10.6 months for pre-treated patients. The 18-month OS rates were 58.2% for treatment-naive patients and 37% for pre-treated patients; 24-month OS rates were 44.5% and 31.3%, respectively. In both cohort of patients, OS increased according to increasing PD-L1 expression (6).
Pembrolizumab was further investigated in KEYNOTE-010, a global, phase II/III trial, which included pre-treated advanced NSCLC patients who had PD-L1 positive tumors (expression ≥1%) (25). A total of 1,034 patients were randomized to receive pembrolizumab at two doses: 2 mg/kg (n=345) or 10 mg/kg (n=346) every 3 weeks, or docetaxel at 75 mg/m2 every 3 weeks (n=343). The primary endpoints were OS and PFS in patients with PD-L1 ≥1% and ≥ 50%. Most patients were former or current smokers. In the total population, median OS was improved in the two pembrolizumab doses (10.4 months for 2 mg/kg; 12.7 months for 10 mg/kg) as compared to docetaxel (8.5 months). For the overall population, PFS primary endpoint was not met. For patients with PD-L1 ≥50%, both pembrolizumab arms demonstrated significantly improved PFS. Pembrolizumab was better tolerated compared to docetaxel, despite a longer exposure. IRAEs, including pneumonitis, were reported at low rates. There were 3/682 patients (<1%) in the pembrolizumab arm that died due to treatment-related pneumonitis.
PD-L1 ≥50% was reported in 28% of patients (n=633), and the outcomes were superior with pembrolizumab in this subgroup. Median OS was 14.9 months for the pembrolizumab 2 mg/kg group, 17.3 months for the 10 mg/kg group, and 8.2 months for the docetaxel group. This study confirmed the benefits of pembrolizumab in patients with pre-treated metastatic NSCLC with PD-L1 expression ≥1% and expanded approval for this previously treated subset of patients with advanced NSCLC following platinum-doublet chemotherapy.
Atezolizumab
Atezolizumab is an engineered, humanized IgG1 anti-PD-L1 monoclonal antibody that blocks the interaction of PD-L1 with the PD-1 receptor, leading to restoration of anti-tumor T-cell activation and T-cell priming (8,26). By blocking PD-L1 only, the PD-L2–PD-1 interaction is preserved and this is a potential advantage that may minimize autoimmunity (26-28). Atezolizumab is the first approved monoclonal antibody that targets the PD-L1 ligand for patients with metastatic NSCLC in the salvage setting.
Clinical trials in the phase I and II setting have demonstrated that durable responses are achieved in patients with NSCLC treated with atezolizumab. These responses are associated with the degree of PD-L1 expression, which has been determined using a different analysis, incorporating PD-L1 expression not only in the tumor cell, but also in the tumor-infiltrating immune cell using immunohistochemistry (IHC) (8,29-31).
The POPLAR study was a randomized phase II study which included 287 patients with previously treated NSCLC (32). Patients were treated with atezolizumab 1,200 mg IV every 3 weeks (n=144) or docetaxel 75 IV mg/m2 every 3 weeks (n=143). The primary endpoint was OS in the intention-to-treat (ITT) population and PD-L1 subgroups. PD-L1 was evaluated on tumor cells and tumor-infiltrating immune cells with the VENTANA SP142 PD-L1 IHC assay (Vantaa Medical Systems, Tucson, AZ, USA). This study met its primary endpoint; with atezolizumab demonstrating an improved OS of 12.6 months versus docetaxel of 9.6 months. The median duration of response in the atezolizumab arm of 14.3 months, versus 7.3 months in the docetaxel arm, confirming the durability of these responses in the immunotherapy subgroup. There was no difference in OS in patients with tumors with negative PD-L1 in both treatment arms. Median OS was improved with atezolizumab (15.5 months) versus docetaxel (9.2 months) with any degree of PD-L1 expression (on either tumor cells or tumor infiltrating cells). The rates of drug-related grade 3/4 AEs were 11% for atezolizumab, compared to 39% for docetaxel. Grade 5 AEs (<1%) were also less frequent than those with docetaxel (2%).
In the phase III OAK trial, patients received atezolizumab at 1,200 mg IV every 3 weeks (n=425) or docetaxel 75 IV mg/m2 (n=425) in a randomized fashion (ITT population) (33). Co-primary endpoints were OS in the ITT and PD-L1-expression subgroup (≥1% PD-L1 on tumor cells or tumor infiltrating immune cells). The primary endpoint was met in this study with improved OS with atezolizumab in the ITT and PD-L1 positive subgroups. The median OS was 13.8 months in the atezolizumab arm versus 9.6 months in the docetaxel arm. The OS in the TC (tumor cell) 1/2/3 or IC (immune cell) 1/2/3 population was superior with atezolizumab (n=241) versus docetaxel (n=222). Patients in the PD-L1 low/negative subgroup (TC0 and IC0) did have superior survival with atezolizumab in this study. Atezolizumab was well tolerated, demonstrated to have lower rates of drug-related grade 3/4 AEs with atezolizumab (15%) versus docetaxel (43%). In the docetaxel arm, one death due to respiratory tract infection attributed to docetaxel was reported. No deaths were reported in the atezolizumab arm.
Both the POPLAR and OAK studies showed that atezolizumab treatment led to superior survival independent of PD-L1 and histology (squamous and non-squamous) and durable responses. This is the first agent targeting PD-L1 that has received approval for previously treated patients with advanced NSCLC following platinum-doublet chemotherapy.
Immune check point inhibitors in development
Durvalumab is an engineered, humanized IgG1 anti-PD-L1 monoclonal antibody. It also spares the interaction between PD-1 receptor and PD-L2, a factor that may be important to minimize autoimmunity (34).
In the phase I/II multicenter study that included those with multiple solid tumors, including NSCLC (35,36), durvalumab generated durable responses in the subgroup with advanced NSCLC, with tolerable side effects. ORR with durvalumab 10 mg/kg IV every 2 weeks was 23% in patients with PD-L1 positive tumors and 5% in patients with PD-L1 negative tumors. PD-L1 was analyzed using Ventana PD-L1 IHC (SP263). Those whose tumor express PD-L1 had superior ORR and OS (36). Serious AEs were seen in 6% of patients. Pneumonitis (Grade 1–2) occurred in 2 (1%) patients. There are several studies investigating durvalumab with or without the CTLA-4 inhibitor (cytotoxic T-lymphocyte-associated antigen-4 inhibitor) tremelimumab in NSCLC in the front-line setting (i.e., MYSTIC: NCT02453282; POSEIDON: NCT03164616)
Avelumab is a fully human IgG1 anti-PD-L1 monoclonal antibody and retains the native Fc region enabling antibody-dependent cell-mediated cytotoxicity (ADCC). The latter has been demonstrated to be advantageous in the pre-clinical setting given the ADCC mediated tumor cell lysis, and this may be an important feature in the clinical setting to enhance the activity of avelumab in combination with vaccines or other immunotherapeutic agents (37). Avelumab was shown to have durable activity in an early phase trial in treatment-naïve patients with NSCLC regardless of PD-L1 (38). Of the total of 145 patients on study, more than half had adenocarcinoma histology. In 75 patients with longer follow-up, ORR was 19% (1 complete response; with 12 ongoing responses) and 45% had disease stability for a disease control rate (DCR) reported at 64%. Avelumab is currently being evaluated in pre-treated advanced NSCLC in a phase III trial that randomizes patients to avelumab or docetaxel (JAVELIN Lung 200; NCT02395172).
Immunotherapy in patients with advanced NSCLC with actionable genomic alterations
Evidence regarding the role of immune checkpoint inhibitors in NSCLC with actionable genomic alterations such as EGFR-mutated or ALK rearranged lung cancers is limited (39,40).
Murine models have demonstrated significant response to the treatment with immune check point inhibitors in EGFR-mutant but not KRAS-driven lung tumors (40). However, in a retrospective study of 58 patients with NSCLC that received immune check point inhibitor therapy, only 4% of patients harboring EGFR mutations or ALK rearrangements were responders and 23% with EGFR negative and ALK-negative or unknown mutations status were responders (39).
In a meta-analysis that included the three studies that compared immune check point inhibitors (nivolumab, pembrolizumab, atezolizumab) to docetaxel (22,25,33), immunotherapy agents significantly improved OS compared to docetaxel, and also specifically in the EGFR negative subgroup. However, this benefit was not seen in the EGFR-mutated sub-group. One explanation for this is that EGFR-mutated lung cancers have low mutation burden, which has been correlated with lower chance of response to immunotherapy. This was substantiated by another study that documented EGFR positive lung cancer to have low mutation burden when analyzed with next-generation sequencing (41).
In the cases of EGFR mutated or ALK rearranged NSCLC, appropriate targeted therapy is the first-line therapy, and upon development of resistance and progression, if no actionable mutation (i.e., osimertinib for T790M), the next line of therapy to be considered is platinum-doublet chemotherapy.
Is PD-L1 the optimal predictive biomarker?
The success of immune check point inhibitors is strongly dependent on optimal patient selection. Developing validated biomarkers that identify patients that will truly benefit from these antibodies remains an area of active investigation (42). Although a subset of patients with NSCLC benefit from PD-1 blockade therapy, many patients do not achieve significant benefit. The mechanisms by which PD-1 blockade modulates the immune system in patients with advanced NSCLC is not fully understood, and there is a critical need to further investigate factors that determine clinical responses to immunotherapy. PD-L1 on tumor cells and on tumor infiltrating cells has been correlated with clinical responses and improved survival to immune check point inhibitors in landmark studies (22,24,25,33).
Both KEYNOTE 001 (24) and KEYNOTE 010 (25) showed that PD-L1 can be applied to select patients for treatment with pembrolizumab. In KEYNOTE 010, patients with PD-L1 ≥1% derived survival benefit from pembrolizumab. The PD-L1 IHC assay using the 22C3 clone reported in KEYNOTE-010 was previously validated and received approval by the FDA to be used as a companion diagnostic test to assist in patient selection for treatment with pembrolizumab (43).
However, in the CheckMate trials, the impact of PD-L1 as a predictive biomarker was not clear (21,22). The CheckMate trials used the Dako 28-8 PD-L1 IHC assay and categorized PD-L1 expression at 3 cut-offs: ≥1%, ≥5%, or ≥10%. In CheckMate 017, PD-L1 expression was not predictive of ORR or OS. In CheckMate 057, PD-L1 expression was predictive for benefit with nivolumab, similarly to what has been previously reported in the phase I study. No significant difference was reported in OS in those with PD-L1 negative tumors between the nivolumab and docetaxel treatment arms. Notably, a significant improvement in survival across the 3 pre-specified cut-off levels of expression (≥1%, ≥5%, or ≥10%) was seen. Based on these results, the Dako 28-8 PD-L1 IHC assay was approved as a complementary diagnostic test, however, it is not required for patient selection for treatment with nivolumab (44).
In the studies investigating atezolizumab, PD-L1 expression was predictive of better outcomes, including OS. PD-L1 assessment with VENTANA SP142 PD-L1 IHC assay was determined based on a score of the percentage of tumor cells (TC3 ≥50%, TC2 ≥5% and <50%, TC1 ≥1% and <5%, and TC0 <1%) and tumor-infiltrating immune cells (IC3 ≥10%, IC2 ≥5% and <10%, IC1 ≥1% and <5%, and IC0 <1%) expressing PD-L1. This method of inclusion of the tumor-infiltrating immune cells is unique to the development of atezolizumab and this finding has not been reported in other PD-1 inhibitor studies (32,33). Nonetheless, this has led to the approval of another diagnostic test for evaluating PD-L1 expression, in this case, as a complementary diagnostic test for atezolizumab, and it is not required for patient selection for treatment with atezolizumab.
The approval of multiple PD-L1 IHC assays to identify the optimal therapies within the currently available immunotherapy agents poses a unique challenge with respect to clinical application of PD-L1 testing and treatment decision making. In the treatment-naive setting, pembrolizumab is approved for patients with advanced NSCLC with PD-L1 ≥50% (using the Dako 22C3 PD-L1 IHC assay). In the salvage setting, pembrolizumab is approved for patients with advanced NSCLC with PD-L1 ≥1%. Both nivolumab and atezolizumab are approved, independent of PD-L1 expression. Given that evaluation of PD-L1 using the 22C3 PD-L1 IHC assay is now standard in the front-line setting, it is unclear how this result can be used to assist in decision making for the second-line setting.
The Blueprint PD-L1 IHC Assay Comparison project was an innovative and collaborative effort that set out to understand the similarities and differences between these four PD-L1 tests (28-8; 22C3; SP142; SP263) and how this could impact results. Three of the four tests (28-8, 22C3, and SP263) demonstrated consistency with regards to tumor cell staining whereas the fourth showed consistently fewer tumor cells stained (45). All of the assays demonstrated immune cell staining, but there was more variation compared to tumor cell staining. This study indicated that despite similar analytical performance of PD-L1 expression for three assays, interchanging assays is not recommended at this time given the risk of inaccurate PD-L1 results for a subset of patients.
Currently, there is ongoing investigation on development of predictive biomarkers in blood or tumor to select patients for greater benefit from immune checkpoint therapy. Studies have demonstrated that mutational burden or gene signatures may be the optimal strategy to help guide treatment decisions (8,46,47).
Our group evaluated peripheral blood of patients with advanced NSCLC receiving immune check point inhibitors at selected time points to determine changes in the peripheral blood T cells and its association with outcomes (42). In responding patients, early PD-L1 positive CD8 T-cell responses were seen following immune check point inhibitor therapy. These proliferating CD8 T cells had an effector-like phenotype which have the potential to generate cytotoxicity. It is of note that this was a transient detection and this is likely followed by an increase of tumor-specific effectors at the site of tumor. We concluded that assessing peripheral blood T cells may be important to identify treatment response and additional studies are needed to confirm these findings.
Immune related adverse side effects (IRAEs)
IRAEs are the most frequent adverse side effects seen with PD-1/PD-L1 inhibitors. In most cases, these adverse side effects are manageable. Patients usually experience these IRAEs within the first 3 months of starting treatment. However, these can occur at any time during treatment and can be seen as a late toxicity, even after discontinuation of therapy (48).
When IRAEs develop, early recognition is critical, with subsequent prompt evaluation and initiation of treatment. Treatment of moderate or severe IRAEs requires holding or discontinuation of the therapy and the use of corticosteroids, and additional immunosuppression, if unresponsive to corticosteroids.
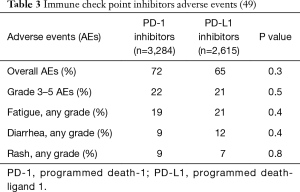
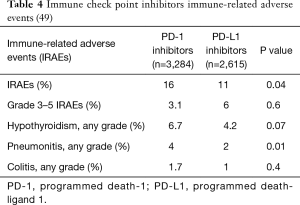
A formal systematic review and pooled analysis was conducted with comprehensive meta-analysis software to evaluate the differences in toxicity of anti-PD-1 versus anti-PD-L1 agents in NSCLC patients (49). There were 12 studies utilizing PD-1 inhibitors and 11 studies with PD-L1 inhibitors between 2013 and 2016. A total of 5,899 patients were evaluated for toxicity, including 3,284 patients treated with PD-1 and 2,615 treated with PD-L1 inhibitors. The rate of overall AEs is similar between PD-1 and PD-L1 inhibitors (72% and 65%, respectively); grade 3 or worse AEs (22% and 21%); or the rates of fatigue, diarrhea, and rash individually. Fatigue was the most common AE, occurring in about one-fifth of patients. Hypothyroidism was the most frequent IRAE reported. There is a slightly higher incidence of IRAEs and pneumonitis with PD-1 inhibitors compared to PD-L1 inhibitors (see Tables 3 and 4).
Future directions
Immune check point inhibitors are being explored in combination strategies with the goal of identifying novel therapies that might increase the ORR and/or overcome resistance in patients who progress following initial therapy. The strategy with regards to optimal combinations and/or sequencing is yet to be defined. Numerous combinations are under investigation, including the combination of the currently approved agents with other agents that target the checkpoint pathway, some that are negative checkpoints (such as CTLA-4, LAG-3, TIM-3) or co-stimulatory agents (such as OX40, GITR), immunomodulatory molecules [such as indoleamide 2,3-dioxygenase (IDO)], targeted therapy or cytotoxic chemotherapy, vaccines, and radiation.
Immune check point inhibitors are also being studied in ongoing clinical trials evaluating the role of immune check point inhibition in earlier stage NSCLC in the pre- (50) or post-operative setting, consolidation therapy after chemoradiation in stage III NSCLC (51), and in relapsed small cell lung cancer (52).
Conclusions
The use of immunotherapy with nivolumab, pembrolizumab, or atezolizumab in the salvage setting comprises standard treatment options in the US. These immune check point inhibitors have led to enthusiasm given the improved outcomes and tolerability compared to docetaxel for patients with advanced NSCLC.
However, despite many reasons for optimism about the potential for research to accelerate the development of highly effective treatments, important challenges remain in defining the subgroup of patients with advanced NSCLC who benefit the most.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Leal served on scientific advisory board meetings for Genentech/Roche, Ariad, Takeda, Astrazeneca and Novartis. Dr. Ramalingam served on scientific advisory board meetings for BMS, Amgen, Abbvie, Astra Zeneca, Lilly, Genentech/Roche, and Merck.
References
- American Cancer Society. Cancer Facts & Figures 2017. Atlanta: American Cancer Society 2017.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013, National Cancer Institute. Bethesda, MD. 2009.
- Leighl NB. Treatment paradigms for patients with metastatic non-small-cell lung cancer: first-, second-, and third-line. Curr Oncol 2012;19:S52-8. [Crossref] [PubMed]
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103. [Crossref] [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [Crossref] [PubMed]
- Ramalingam S, Hui R, Gandhi L, et al. Long-Term OS for Patients With Advanced NSCLC Enrolled in the KEYNOTE-001 Study of Pembrolizumab. J Thorac Oncol 2016;11:S241-2. [Crossref] [PubMed]
- Jemal A, Ward EM, Johnson CJ, et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst 2017.109. [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [Crossref] [PubMed]
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012;24:207-12. [Crossref] [PubMed]
- Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002;99:12293-7. [Crossref] [PubMed]
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012;24:207-12. [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2014;2:846-56. [Crossref] [PubMed]
- Brahmer JR, Drake CJ, Wollner I, et al. Phase I Study of Single-Agent Anti–Programmed Death-1 (MDX-1106) in Refractory Solid Tumors: Safety, Clinical Activity, Pharmacodynamics, and Immunologic Correlates. J Clin Oncol 2010;28:3167-75. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. [Crossref] [PubMed]
- Brahmer JR, Horn L, Jackman D, et al. Five-year follow-up from the CA209-003 study of nivolumab in previously treated advanced non-small cell lung cancer: clinical characteristics of long-term survivors. Presented at: 2017 AACR Annual Meeting. Washington, DC, 2017:Abstract CT077 2017.
- Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Barlesi F, Steins M, Horn L, et al. Long-term outcomes with nivolumab (Nivo) vs docetaxel (Doc) in patients (Pts) with advanced (Adv) NSCLC: CheckMate 017 and CheckMate 057 2-y update. Ann Oncol 2016;27:1215PD.
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy--inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res 2012;18:6580-7. [Crossref] [PubMed]
- Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261-8. [Crossref] [PubMed]
- Akbari O, Stock P, Singh AK, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol 2010;3:81-91. [Crossref] [PubMed]
- Horn L, Spigel DR, Gettinger SN, et al. Clinical activity, safety and predictive biomarkers of the engineered antibody MPDL3280A (anti-PDL1) in non-small cell lung cancer (NSCLC): update from a phase Ia study. J Clin Oncol 2015;33:abstr 8029.
- Spigel DR, Chaft JE, Gettinger SN, et al. Clinical activity and safety from a phase II study (FIR) of MPDL3280A (anti-PDL1) in PD-L1–selected patients with non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:abstr 8028.
- Peters S, Gettinger S, Johnson ML, et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH). J Clin Oncol 2017;35:2781-9. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Stewart R, Morrow M, Hammond SA, et al. Identification and Characterization of MEDI4736, an Antagonistic Anti-PD-L1 Monoclonal Antibody. Cancer Immunol Res 2015;3:1052-62. [Crossref] [PubMed]
- Rizvi NA, Brahmer JR, Ou S-HI, et al. Safety and clinical activity of MEDI4736, an anti-programmed cell death-ligand 1 (PD-L1) antibody, in patients with non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:abstr 8032.
- Antonia SJ, Brahmer JR, Khleif S, et al. Phase 1/2 study of the safety and clinical activity of durvalumab in patients with non-small cell lung cancer (NSCLC). Ann Oncol 2016;27:416-54. [Crossref]
- Statistics NCfH: United States life tables: US decennial life tables for 1979-1981. Washington DC: US Governement Printing Office vol 1, no. 1:85-1150-1, (DHHS publication PHS). 1985.
- Jerusalem G, Chen F, Spigel D, et al. JAVELIN Solid Tumor: Safety and Clinical Activity of Avelumab (Anti-PD-L1) as First-Line Treatment in Patients with Advanced NSCLC. J Thorac Oncol 2017;12:S252. [Crossref]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355-63. [Crossref] [PubMed]
- Spigel DR, Schrock AB, Fabrizio D, et al. Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD-1/PD-L1 targeted therapies. J Clin Oncol 2016;34:abstr 9017.
- Kamphorst AO, Pillai RN, Yang S, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A 2017;114:4993-8. [Crossref] [PubMed]
- U.S. Food and Drug Administration. FDA approves Keytruda for advanced non-small cell lung cancer. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm465444.htm. Accessed October 2, 2015.
- . Accessed February 1,2018.http://wayback.archive-it.org/7993/20170111231625/http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm466576.htm
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol 2017;12:208-22. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Champiat S, Ferté C, Lebel-Binay S, et al. Exomics and immunogenics: Bridging mutational load and immune checkpoints efficacy. Oncoimmunology 2014;3:e27817. [Crossref] [PubMed]
- Gordon R, Kasler MK, Stasi K, et al. Checkpoint Inhibitors: Common Immune-Related Adverse Events and Their Management. Clin J Oncol Nurs 2017;21:45-52. [Crossref] [PubMed]
- Pillai R, Behera M, Owonikoko T, et al. Evaluation of Toxicity Profile of PD-1 versus PD-L1 Inhibitors in Non-Small Cell Lung Cancer (NSCLC). J Thorac Oncol 2017;12:S253-4. [Crossref]
- Chaft JE, Forde PM, Smith KN, et al. Neoadjuvant nivolumab in early-stage, resectable non-small cell lung cancers. J Clin Oncol 2017;35:abstr 8508.
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]