Modeling the relationship between baseline lactate dehydrogenase and prognosis in patients with extensive-disease small cell lung cancer: a retrospective cohort study
Introduction
Small cell lung cancer (SCLC) accounts for approximately 13% in lung cancer patients (1). It’s an aggressive disease characterizing for rapid progression and distant metastases. Most of patients are primarily diagnosed with an extensive disease (ED), of which the total 5-year survival rate is less than 7% (2). Currently, platinum-based combined chemotherapy is the standard treatment for ED-SCLC. Although SCLC is sensitive to the chemotherapy, most of the patients will be suffered from disease progression and distant metastases within 2 years after first-line treatment (3). In recent decades, novel approaches of immunotherapy have brought light to cancer treatment (4,5). And trials for investigating the efficacy and safety of immunotherapy with or without chemotherapy in SCLC patients are ongoing (6,7).
Predicting prognostic risks of SCLC patients through reliable biomarkers can provide information for disease monitoring and treatment evaluation, which will contribute to the exploration of novel treatments. Lactate dehydrogenase (LDH) is the key enzyme in glycolytic pathway, which is the main source of energy in activities of rapid proliferation and distant metastases for tumor cells. When tissue damage occurs because of tumor activities, LDH will be released from intracellular microenvironment and then lead to an abnormal elevation of serum LDH (8). Therefore, measurements of serum LDH levels can reflect the intensity of tumor activities and thus can be used as a prognostic biomarker. Zhang et al. (9) conducted a meta-analysis involving 4,785 SCLC patients from 28 studies to investigate the prognostic value of baseline LDH for SCLC patients. Their results indicated that patients with an abnormal elevation of serum LDH at baseline would have a 1.45 times higher risk of death than those with normal LDH level (HR =1.45; 95% confidence interval (CI), 1.27–1.66). However, measurements of LDH were all treated as binary outcomes in above studies with a cutoff value at the upper limit of normal range. In that way, it would be impossible to explore the continuous relationship between baseline LDH and survival; namely, different LDH levels within the same group would be regarded as to have the same risk of death.
Modeling the relationship between exposure of risk factors and disease outcomes can provide more accurate information for clinical practice. Zhou et al. (10) found nonlinear associations between biomarkers and prognosis in resectable pancreatic ductal adenocarcinoma (PDAC) patients. Their results suggested that a preoperative Carbohydrate antigen 19-9 (CA19-9) level of 100 U/mL and a carcinoembryonic antigen (CEA) level of 10 µg/mL are best cutoff values to predict PDAC prognosis. Kreike et al. (11) identified a “V” shape association between the expression ratio of EGFR gene and the risk of recurrence in breast cancer patients who had received surgery. This result indicated that both lower and higher expression of EGFR would increase the probability of disease recurrence.
To our knowledge, the continuous relationship between baseline serum LDH and prognosis of SCLC patients has not been reported. Therefore, the present study is aimed to reveal how different levels of baseline LDH are associated with patients’ outcome.
Methods
Patients and data collection
SCLC patients who were primarily diagnosed at any tertiary hospital in Shanghai, China from April 2011 to August 2014 were eligible for our study if they met the following criterion: cytologically or histologically confirmed SCLC, clinical staged ED-SCLC patients (according to VALSG staging system or AJCC 7th Edition), age ≥18 years, received chemotherapy within 30 days after primary diagnosis.
Data were retrospectively collected from Shanghai Health Information Network, which is organized and funded by Shanghai Municipal Commission of Health and Family Planning. Demographic data, diagnosis and staging information, chemotherapy and surgery information and laboratory testing data [including LDH, CEA and albumin (ALB)] were extracted from the database. Measurements of biomarkers were uniformly scaled by linear standardization to balance small differences of testing limits among hospitals. Patients’ survival outcomes were checked by classified resident identification number through Medical Insurance Bureau of Shanghai.
Statistical analysis
A multivariate Cox proportional hazard model was used to detect the association between baseline serum LDH and overall survival, adjusting for age, gender, CEA and albumin. The corresponding continuous relationship was analyzed by using natural spline functions in the Cox model. LDH data were logarithmic transformed before analysis. Likelihood ratio (LR) test was used to examine the goodness of fit for this composite model. An appropriate cutoff value of LDH level was selected by the shape of the curve to explore its clinical significance. Log-rank test was used to compare the survival between different groups. Subgroup analyses were performed under different levels of CEA and ALB. All analyses were performed by SAS v9.4 (Inc., Cary, NC) and packages of “splines” and “akima” in R software.
Results
Patients’ screening and baseline characteristics
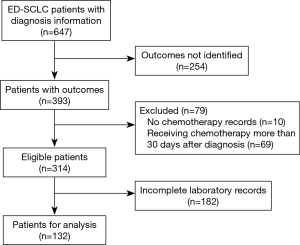
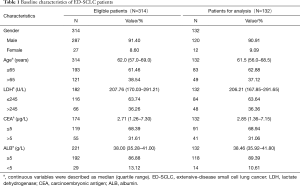
A total of 647 ED-SCLC patients were identified in the database and 314 of them were considered eligible for our study according to inclusion criterion. Among the 314 eligible patients, 132 of them had complete measurements of baseline LDH, CEA and ALB and thus were used for analyses (Figure 1). The baseline characteristics of these patients were listed in Table 1. In general, conditions were similar between 314 eligible patients and those used for analyses. About 90% of these ED-SCLC patients were male and they were mainly composed of middle-aged and elderly people. Most of the patients had a normal ALB level at baseline, while there were 36% and 31% of the patients had elevated levels of LDH (>245 U/L) and CEA respectively. Patients’ outcomes were checked in March 2016. The median overall survival time of 132 analyzed ED-SCLC patients was 295 days.
Full table
Multivariate analysis
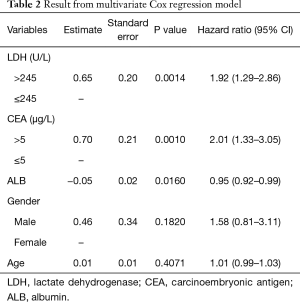
Results of multivariate Cox regression model were shown in Table 2. Elevated levels of LDH and CEA at baseline were identified to be risk factors of survival for ED-SCLC patients. Patients with an abnormal baseline LDH level would have a 1.92-fold higher risk of death than those with normal LDH levels (95% CI, 1.29–2.86). Similarly, an elevated CEA level at baseline would double the risk of death for ED-SCLC patients (HR =2.01; 95% CI, 1.33–3.05). Besides, One-unit increase of ALB was found to reduce the risk of death by 5% (HR =0.95; 95% CI, 0.92–0.99). Age and gender were not significantly associated with survival in our study.
Full table
Modeling continuous relationship
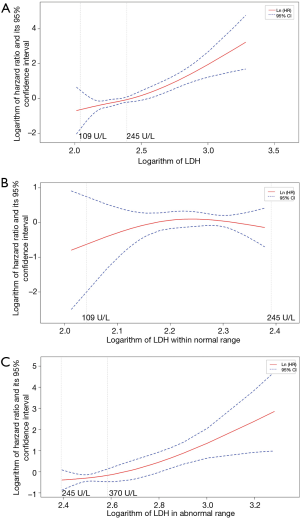
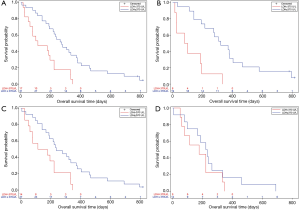
Figure 2A showed the association between different levels of baseline LDH and patients’ prognosis, which was fitted by using natural spline functions in a univariate Cox model. The curve revealed that the risk of death would keep rising with the increase of baseline LDH level in general. Result of LR test approved the significant contribution of these spline functions to model fitting (P<0.0001). Lower LDH levels showed a trend for representing lower risks in patients with normal LDH levels (109–245 U/L) at baseline (Figure 2B). But the confidence interval of logarithm hazard ratio contained zero throughout this range. However, when referred to patients with elevated levels of LDH (>245 U/L), we found that the increment of baseline LDH at this high level would still result in the rise of prognostic risk (P value of LR test, 0.0044). We chose 370 U/L as cutoff value and then compared survival between two subgroups divided, namely patients with a baseline LDH level of 245–370 U/L, or with a LDH level of greater than 370 U/L. As Figure 2C showed, patients with a relatively higher baseline LDH level (>370 U/L) would have a poorer survival when compared with those who had moderately elevated LDH levels (median survival time, 114 vs. 274 days; P value of log rank test, 0.007).
Subgroup analyses were performed among those patients with elevated LDH levels to further detect the clinical significance of above cutoff value (Figure 3). For patients with a normal CEA level at baseline, LDH level higher than 370 U/L still represented for a poorer survival (P=0.0005). Results were similar for patients with sufficient ALB at baseline (P=0.0094). However, this significance was not observed in patients who had abnormally elevated CEA level at baseline (P=0.2968). It was unable to analyze the subgroup of hypoalbuminemia patients due to the small sample size.
Discussion
As an important prognostic factor for SCLC patients, many researches have revealed the association between elevated LDH levels at baseline and poor survival (9,12). In the present study, patients who had an abnormally elevated baseline LDH were observed to have a 1.92-fold risk of death than those with normal LDH (95% CI, 1.29–2.86). These results are consistent with the estimates reported by Bremnes et al. (13) and Karachaliou et al. (14) for ED-SCLC subgroups (HR =2.10; 95% CI, 1.50–2.80; and HR =1.92; 95% CI, 1.56–2.44 respectively). Results of multivariate Cox regression also suggested that an elevated serum CEA was associated with a poor prognosis, while a sufficient ALB level at baseline could reduce the risk of death. An increase in serum CEA level at baseline can be observed in several malignant diseases and is not informative in screening and diagnosis of SCLC. But it has been reported that an elevated CEA level during the treatment duration of SCLC could precede a relapse weeks to months before clinical signs occur, thus can be used in disease monitoring (15,16). Serum ALB level reflects the general nutrition status for patients with malignant diseases. Several studies have been conducted by Zhang and his group to demonstrate the prognostic value of ALB and its composite indexes (e.g., the prognosis nutritional index, PNI) for SCLC patients (17-19).
Besides, the present study is the first study to investigate the continuous relationship between baseline serum LDH and survival of SCLC patients. Our results revealed that the risk of death would keep rising with the increase of baseline LDH level. Among patients with an abnormally elevated LDH level, those who had relatively higher LDH levels (>370 U/L) would have a poorer survival than those with moderately elevated LDH levels (245–370 U/L). This finding indicates that despite the upper limit of normal range, there could still be differences in survival among patients with abnormal LDH levels. However, the change of LDH level did not significantly affect the risk of death within normal range.
Subgroup analyses showed that the LDH level of 370 U/L was an effective cutoff value to predict prognosis among patients who had a normal CEA level or a sufficient ALB level at baseline. Conversely, the difference in survival between two LDH subgroups (>370 U/L and 245–370 U/L) was not significant in patients with an elevated baseline CEA level. This may be explained as that the prognostic value of a fairly high LDH level might be weakened when patients already have another abnormal biomarker that raises the risk of death.
The main limitations of our study are retrospective data collection and missing data. We were not able to assess the baseline disease status for included patients due to inadequate information of corresponding variables, such as ECOG score and complication. And this may lead bias into our results. Moreover, many eligible ED-SCLC patients were not included in data analysis since their laboratory measurements were incomplete. Despite this we may not have to exaggerate the impact of missing data for that the baseline characteristics are similar between 314 eligible patients and 132 analyzed patients. Besides, there were chances that we chose 370 U/L as the cutoff value of LDH to detect differences in survival between patient subgroups divided by this limit. Further studies are required to confirm the prognostic value of above LDH level.
In conclusion, we have explored the continuous relationship between baseline serum LDH and survival in patients with ED-SCLC. Our study has revealed that even in patients with an abnormal elevated LDH at baseline, a fairly higher LDH level (LDH >370 U/L) could still refer to a poorer survival. These results suggest that it may not be adequate to predict patients’ prognosis through biomarkers only by their limits of normal range. The interpretation of continuous relationship between biomarkers and survival would provide more accurate information for clinical practice.
Acknowledgements
Funding: This study was supported by the National Natural Science Foundation of China (number 81273187) and School of Public Health and Key Laboratory of Public Health Safety. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of Interest to declare.
Ethical Statement: Patient data were retrieved from Shanghai Health Information Network. Residential identification numbers were classified by MD5 encryption for privacy.
References
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011;378:1741-55. [Crossref] [PubMed]
- Bernhardt EB, Jalal SI. Small Cell Lung Cancer. Cancer Treat Res 2016;170:301-22. [Crossref] [PubMed]
- Gardner EE, Lok BH, Schneeberger VE, et al. Chemosensitive Relapse in Small Cell Lung Cancer Proceeds through an EZH2-SLFN11 Axis. Cancer Cell 2017;31:286-99. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Spigel DR, Socinski MA. Rationale for chemotherapy, immunotherapy, and checkpoint blockade in SCLC: beyond traditional treatment approaches. J Thorac Oncol 2013;8:587-98. [Crossref] [PubMed]
- Reck M, Heigener D, Reinmuth N. Immunotherapy for small-cell lung cancer: emerging evidence. Future Oncol 2016;12:931-43. [Crossref] [PubMed]
- Augoff K, Hryniewicz-Jankowska A, Tabola R. Lactate dehydrogenase 5: An old friend and a new hope in the war on cancer. Cancer Lett 2015;358:1-7. [Crossref] [PubMed]
- Zhang X, Guo M, Fan J, et al. Prognostic significance of serum LDH in small cell lung cancer: A systematic review with meta-analysis. Cancer Biomark 2016;16:415-23. [Crossref] [PubMed]
- Zhou G, Liu X, Wang X, et al. Combination of preoperative CEA and CA19-9 improves prediction outcomes in patients with resectable pancreatic adenocarcinoma: results from a large follow-up cohort. Onco Targets Ther 2017;10:1199-206. [Crossref] [PubMed]
- Kreike B, Hart G, Bartelink H, et al. Analysis of breast cancer related gene expression using natural splines and the Cox proportional hazard model to identify prognostic associations. Breast Cancer Res Treat 2010;122:711-20. [Crossref] [PubMed]
- Harmsma M, Schutte B, Ramaekers FC. Serum markers in small cell lung cancer: Opportunities for improvement. Biochim Biophys Acta 2013;1836:255-72. [PubMed]
- Bremnes RM, Sundstrom S, Aasebo U, et al. The value of prognostic factors in small cell lung cancer: results from a randomised multicenter study with minimum 5 year follow-up. Lung Cancer 2003;39:303-13. [Crossref] [PubMed]
- Karachaliou N, Papadaki C, Lagoudaki E, et al. Predictive value of BRCA1, ERCC1, ATP7B, PKM2, TOPOI, TOPOmicron-IIA, TOPOIIB and C-MYC genes in patients with small cell lung cancer (SCLC) who received first line therapy with cisplatin and etoposide. PLoS One 2013;8:e74611. [Crossref] [PubMed]
- Bandoh S, Fujita J, Ueda Y, et al. Expression of carcinoembryonic antigen in peripheral- or central-located small cell lung cancer: its clinical significance. Jpn J Clin Oncol 2001;31:305-10. [Crossref] [PubMed]
- Molina R, Auge JM, Escudero JM, et al. Mucins CA 125, CA 19.9, CA 15.3 and TAG-72.3 as Tumor Markers in Patients with Lung Cancer: Comparison with CYFRA 21-1, CEA, SCC and NSE. Tumour Biol 2008;29:371-80. [Crossref] [PubMed]
- Zhou T, He X, Fang W, et al. Pretreatment Albumin/Globulin Ratio Predicts the Prognosis for Small-Cell Lung Cancer. Medicine (Baltimore) 2016;95:e3097. [Crossref] [PubMed]
- Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol 2015;36:3389-97. [Crossref] [PubMed]
- Zhou T, Zhan J, Hong S, et al. Ratio of C-Reactive Protein/Albumin is An Inflammatory Prognostic Score for Predicting Overall Survival of Patients with Small-cell Lung Cancer. Sci Rep 2015;5:10481. [Crossref] [PubMed]