Telemonitoring of left-ventricular assist device patients—current status and future challenges
Introduction
Steady technical improvement in the left-ventricular assist devices (LVADs) being implanted today has led to increasingly good clinical results (1-8), with assist times of over 10 years no longer being exceptional (9). What remains, however, is a significant number of severe complications, with a high rate of readmission to hospital in the long term (10-16). The most frequent complications are renewed heart failure (HF), thromboembolism, haemorrhage, infection (especially driveline infections) and right-HF (10-16).
Aftercare following the inpatient stay usually comprises visits to the outpatient department of the implantation centre approximately every 3 months (17). Between visits, the quality of the aftercare largely depends on patient compliance and self-management (18). During the long phases between outpatient visits, telemonitoring is a good way of monitoring patients intensively and with the involvement of physicians (19).
The term telemonitoring covers all manner of applications for the electronic transfer of patient biological data or self-reports to a clinical physician. Typical biological data might include heart rate, blood pressure, ECG changes, oxygen saturation, body weight, breathing rate and body temperature. Telemonitoring has so far been used most frequently in patients with chronic HF. In this field we therefore have the broadest set of data currently available (20-22). A potential to improve the care, quality of life and prognosis of patients with chronic diseases has been ascribed to telemonitoring, and yet the potential of telemonitoring in conjunction with LVAD patients has only been exploited sparingly to date (23-26). It is precisely this patient group which opens up completely new telemonitoring possibilities, however. In addition to biological data and self-reports, there is also potentially the option to transmit meaningful parameters from the LVAD itself and any other implanted devices, as well as photographs of the driveline exit site.
This paper summarizes the different options which exist in the context of LVAD telemonitoring to date, and also describes the complex requirements for adequate telemonitoring of LVAD patients in a bid to facilitate the advance of this form of monitoring to a standard procedure in the near future.
Telemonitoring of relevant parameters in LVAD patients
Telemonitoring can be conducted without hardly any active patient participation (e.g., through implants) or with active patient participation (e.g., measurement of INR).
In order for the telemonitoring of LVAD patients to be comprehensive and make sense, the authors are of the opinion that physicians should have continual access to the following parameters:
- LVAD controller parameters (alarms, rotary speed, power consumption, flow, pulsatility index);
- Blood pressures (pulmonary artery pressure, mean arterial pressure);
- Pacemaker [cardiac resynchronisation therapy (CRT)/implantable cardioverter defibrillator (ICD)]-related parameters (e.g., heart rhythm, thoracic impedance);
- Coagulation values (INR) and medication;
- Further smartphone-transmitted parameters and findings (photos of driveline exit site, body-related data, activity).
LVAD controller parameters (alarms, rotary speed, power consumption, flow, pulsatility index)
The continual monitoring of pump parameters, especially power consumption, is an extremely important component of telemonitoring in LVAD patients. Changes in power consumption can be an indication of severe complications, such as pump thromboses or uncontrolled and steeply elevated blood pressure levels. Unfortunately, continual monitoring of parameters from the two most frequently implanted LVAD pump systems (HeartMate II or III, Thoratec, Corp., Pleasanton, CA, USA and HeartWare, Inc., Framingham, MA, USA) is not currently possible. Some of the necessary interfaces are already integrated in the devices, but the interfaces have not been cleared for telemonitoring due to the prohibition of remote treatment, the detectability of medical services, as well as liability issues (on the part of the treating physicians, but also on that of the manufacturers regarding technical defects) (27-29).
Clinical experience with the remote monitoring of LVAD patients is only available in conjunction with the HeartAssist 5® system (MicroMed Cardiovascular, Inc., Houston, TX, USA). The HeartAssist 5® permits reliable access to both real-time and historic pump parameters, and alarms can be sent to any computer or smartphone. It facilitates the monitoring of current flow curves and the graphic presentation of stored data in the formats: 4 hours, 24 hours, 7 days and 30 days.
First experiences with this system were published by Pektok et al. (23), who observed 5 patients with a median follow-up of 253 days. In three of the five patients, alarms were activated. The most frequent were low-flow alarms, which led to a corresponding management of fluid balance. One patient was admitted to hospital with a suspected pump thrombosis after an “excess power” and a “pump stopped/restarted” alarm. On the ward, thrombosis could then be excluded by echocardiographic and chemical laboratory testing. The anticoagulation therapy was then optimised.
Overall, the authors conclude that remote monitoring is a helpful tool for the early detection of serious problems and their timely treatment. They point out that each patient has a different alarm threshold, and that the threshold should be adjusted to the current haemodynamic conditions on every visit to hospital. They believe, however, that the other advantages of remote monitoring (e.g., impact on the mental well-being of patients) need to be evaluated in larger studies. Despite all its potential advantages, they see remote monitoring as complementary to the aftercare currently in existence. It should not replace routine outpatient visits. Ultimately, it has to be said that the HeartAssist 5® has far fewer applications than the two market leaders, and that therefore its potential advantages could not yet be evaluated in larger studies.
Blood pressures (pulmonary artery pressure, mean arterial pressure)
Pulmonary artery pressure
In patients with chronic left-HF, pulmonary vascular resistance increases, and thus also pulmonary artery pressure increases. Pulmonary artery pressure is in itself dynamic. A previously increased value can fall under LVAD support, sometimes to within the normal range. Just how pulmonary artery pressure will develop after implantation is, however, difficult to predict (30).
The continual measurement of pulmonary artery pressure in LVAD patients is therefore hugely important, also in order to monitor therapy with pulmonary artery pressure-sinking medication. Moreover, the measurement of pulmonary artery pressure helps with fluid management. Performing a conventional right-heart catheter examination at short intervals to determine pulmonary artery pressure in an anticoagulated patient is extremely laborious and not without risks for the patient.
The CardioMEMS™ HF system (St. Jude Medical, Inc., Saint Paul, Minnesota, USA) uses a miniaturized, wireless monitoring sensor which is implanted in the pulmonary artery during a minimally invasive intervention in order to measure pulmonary artery pressure directly (31,32) (Figure 1).

The external measuring system tracks wirelessly and uses the data to determine pulmonary artery pressure. At home, the HF patient uses a portable electronic unit and a special cushion with an antenna to retrieve sensor values once a day. This process is very simple and takes just a few minutes. The electronic unit is switched on and the patient lies down on the cushion. The physician accesses the pressure parameters and trend data of the patient via a patient management website, and this valuable clinical information can be used as orientation when making treatment decisions. If the pressure parameters are outside certain predefined ranges, an alarm is automatically sent to the physician.
Despite the clear clinical evidence, use of the CardioMEMS system in LVAD patients has only been reported in very small patient groups to date. Guglin et al. (26) reported use of the CardioMEMS system in four LVAD patients. The authors could show that in three of the four patients the diastolic pulmonary artery pressure could be reduced after just a few months of monitoring. In addition, all four patients had fewer readmissions to hospital after CardioMEMS implantation, as well as improvements in their serum-creatinine values.
Mean arterial pressure
Equally unsatisfactory is the monitoring of arterial blood pressure in a domestic environment. The currently implanted left-heart support systems with their continuous flow are not compatible with conventional blood pressure measurement. Measurement with Doppler technology is recommended, especially when there is no palpable pulse. Whether the systolic or the mean arterial pressure should then be measured is currently a matter of controversial debate. Giving each patient a portable Doppler device is not yet a viable option because of availability and also because of the costs involved. This means that telemonitoring of blood pressure values will not be realizable in the near future for the simple reason that patients are unable to measure their blood pressure. This is particularly regrettable with regard to the fact that increased mean arterial pressure in LVAD patients leads to a significant occurrence of severe complications (stroke, haemorrhage and progressive aortic valve insufficiency) (33).
Pacemaker (CRT/ICD)-related parameters (e.g., heart rhythm, thoracic impedance)
The IN-TIME study (34) showed that total mortality in HF patients can be reduced by more than 50% through implant-based home monitoring, independently of whether patients receive an ICD or an ICD with CRT-D.
Many LVAD patients are recipients of an implanted defibrillator with or without cardiac resynchronisation function (ICD, CRT-D). In the HeartMate II trial, the proportion was 82%, in the HeartMate II registry 75%. Thus, in the majority of LVAD patients the additional possibility exists to generate extra information (both patient- and device-related) via their pacemaker systems. Besides heart rhythm, thoracic impedance should also be particularly significant for these patients (35,36).
Thoracic impedance is the result of measuring electrical resistance between the right-ventricular electrode and the aggregate housing. Thoracic impedance provides information about intrathoracic fluid status, with a reduction in thoracic impedance indicating increasing fluid collection in the lung. Changes in thoracic impedance often occur days before the first clinical symptoms.
Most manufacturers have developed their own remote monitoring system, with its own platform. They are:
- Home MonitoringTM (Biotronik, SE & Co. KG, Berlin, Germany);
- CareLink NetworkTM (Medtronic, Inc., Minneapolis, MN, USA);
- Latitude Patient Management systemTM (Boston Scientific, Corp., St Paul, USA);
- Merlin.netTM (St Jude Medical, LLC, Sylmar, USA).
The data captured by the ICD are transferred to a central server, either via an analogue landline telephone and a free phone number (Boston Scientific, Medtronic und St-Jude Medical Systems) or via a GSM network (Biotronik). The data are then processed and made accessible to treating physicians on a secure website. Physicians are alarmed by email, SMS, fax or phone if any of the data sent are critical. The events which trigger an alarm can be adjusted from patient to patient. First indications of a deterioration in, for example, cardiac haemodynamics, can thus be detected early, which is particularly relevant in conjunction with HF. It is then possible to react quickly to any changes and to counter them with treatment.
At the present moment, the possibilities for monitoring LVAD patients using pacemaker systems are hardly exploited at all. One obstacle is the abovementioned variety of home monitoring platforms. For treating physicians, it is extremely laborious to log into the platform of a different manufacturer for each LVAD patient in order to access the relevant parameters.
Coagulation values (INR) and medication
Close-meshed INR monitoring is highly significant for all LVAD patients with regard to thromboembolic or haemorrhage-related complications (37). For this reason, shortly after implantation patients receive detailed training in INR self-management (Coaguchek, Roche diagnostics, Switzerland). The advantages of INR self-management compared to conventional monitoring by a general practitioner have already been sufficiently proven for other indications (artificial heart valves) (38). In order to support patients with their INR self-management or, if required, adjustment of their Coumadin medication, first attempts have already been made to transfer measured INR values to the hospital via the Vitaphone remote tele-platform, and to send an alarm to the physician if values are outside the target range (25). Wide application of this has yet to follow.
Further smartphone-transmitted parameters and findings (photos of driveline exit site, body-related data, activity)
One of the most-feared complications in LVAD treatment, especially in the long term, is driveline infection (39). Treatment of a driveline infection is usually complex and lengthy. It often ends in surgical restoration, in the worst case with a pump exchange (40). This makes prevention of driveline infection particularly important.
The transfer, analysis and subsequent evaluation of dermatological images are well known from the field of teledermatology. Dermatological changes are easy to visualize using digital cameras and smartphones. For this reason, changes to the skin are particularly well suited to telemedicine. Photographic documentation of the driveline exit site takes place routinely at every outpatient presentation. Within the framework of a research project, the authors of this paper are planning smartphone transmission of photos of the driveline exit site to the hospital (41). An algorithm based on image pixelation is currently being developed to detect inflammations around the driveline exit site early on (Figure 2).
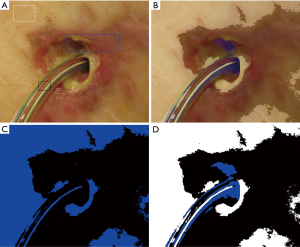
Further possibilities for smartphone use are provided by mobile apps. They allow LVAD patients to convey various parameters (e.g., INR) and body-related data (weight, colour of urine, stool consistency, etc.) every day.
Finally, a smartphone can also be used as an activity tracker, to monitor from the hospital the daily mobility of patients. Performance parameters are defined in the hospital and should correlate with patient mobility following discharge from the hospital. If any significant changes in mobility should occur, physicians can enquire into the reasons without delay (42).
Future challenges
The development and implementation of telemedical applications still faces key challenges: compatibility with the prohibition of remote treatment, delegability of medical services and liability issues (on the medical, but also the technical side), as well as adequate consideration of informational self-determination in the areas of information safety and data protection, or the joint creation of quality standards (27-29).
Potentially, telemonitoring offers a wide range of possibilities, particularly in the aftercare of LVAD patients. Pump parameters are not transmitted today because the required interfaces, some of which are already integrated in the devices, have not been cleared for use by the manufacturers. The abovementioned factors are probably the main reason for this. A solution in the short term would be desirable, especially for patients who live a long distance from their implantation centre.
Sensors to measure blood pressure (especially CardioMEMS to measure pulmonary artery pressure) have only been implanted in extremely few cases to date. This could well be due to the somewhat laborious capturing of data on a special cushion, but also to the lack of financial reimbursement. Telemedical monitoring of arterial blood pressure is currently impossible for the simple reason that the problem of how to measure mean arterial blood pressure in a domestic environment has yet to be solved. Alternatives to measurement by Doppler technology need to be evaluated in the short term (43).
It is also regrettable that monitoring via simultaneously implanted pacemaker systems is a possibility which is not yet consistently exploited. Here the development of a single platform, into which the different manufacturer platforms can be integrated, should definitely be promoted. Physicians need the data from all their LVAD patients to be accessible on a single platform in order to avoid lengthy logging in to various home monitoring platforms or jumping around between one platform and the next.
At the moment the simplest thing to implement in the everyday lives of LVAD patients seems to be the use of smartphones and mobile apps. Parameters and data concerning the health of LVAD patients can be transmitted to hospital quickly and easily (Figure 3).
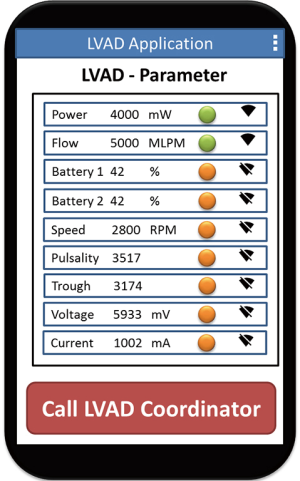
Overall, telemonitoring of LVAD patients has great potential, even the possibility of monitoring more parameters and data than in hardly any other patient group. And the consequences of a timely detection of complications are more significant in the group of LVAD patients than in any other patient collective. Rapid detection of a developing pump thrombosis, for example, not only saves the patient from a repeat surgical intervention and pump exchange, but also leads to considerable financial savings for the healthcare system.
At the present time, exploitation of the potential of telemonitoring in LVAD patients is rudimentary. Here enormous progress is required so that the data captured can be presented to physicians in a clear and straightforward manner. Only then can the telemonitoring of LVAD patients become an instrument in the aftercare of this extremely individual and complex patient group which makes sense.
Acknowledgements
Funding: This project is funded by the German Federal Ministry of Education and Research (BMBF) within the framework of the ITEA 3 Project Medolution [14003].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hanke JS, Rojas SV, Dogan G, et al. First series of left ventricular assist device exchanges to HeartMate 3. Eur J Cardiothorac Surg 2017;51:887-92. [Crossref] [PubMed]
- Rojas SV, Avsar M, Uribarri A, et al. A new era of ventricular assist device surgery: less invasive procedures. Minerva Chir 2015;70:63-8. [PubMed]
- Rojas SV, Avsar M, Hanke JS, et al. Minimally invasive ventricular assist device surgery. Artif Organs 2015;39:473-9. [Crossref] [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Minimally-invasive valve surgery. J Am Coll Cardiol 2010;56:455-62. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Avsar M, et al. Minimally-invasive LVAD Implantation: State of the Art. Curr Cardiol Rev 2015;11:246-51. [Crossref] [PubMed]
- Egger C, Schmitto J, Roth P, et al. How to Shoot the Parachute-Minimally Invasive Ventricular Assist Device Surgery in a Patient Wearing All Existing Cardiologically Available Interventions. Artif Organs 2017;41:683-5. [Crossref] [PubMed]
- Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant 2010;29:S1-39. [Crossref] [PubMed]
- Gustafsson F, Rogers JG. Left ventricular assist device therapy in advanced heart failure: patient selection and outcomes. Eur J Heart Fail 2017;19:595-602. [Crossref] [PubMed]
- Pinney SP, Anyanwu AC, Lala A, et al. Left Ventricular Assist Devices for Lifelong Support. J Am Coll Cardiol 2017;69:2845-61. [Crossref] [PubMed]
- Akhter SA, Badami A, Murray M, et al. Hospital Readmissions After Continuous-Flow Left Ventricular Assist Device Implantation: Incidence, Causes, and Cost Analysis. Ann Thorac Surg 2015;100:884-9. [Crossref] [PubMed]
- Forest SJ, Bello R, Friedmann P, et al. Readmissions after ventricular assist device: etiologies, patterns, and days out of hospital. Ann Thorac Surg 2013;95:1276-81. [Crossref] [PubMed]
- Haglund NA, Davis ME, Tricarico NM, et al. Readmissions After Continuous Flow Left Ventricular Assist Device Implantation: Differences Observed Between Two Contemporary Device Types. ASAIO J 2015;61:410-6. [Crossref] [PubMed]
- Hasin T, Marmor Y, Kremers W, et al. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol 2013;61:153-63. [Crossref] [PubMed]
- Hernandez RE, Singh SK, Hoang DT, et al. Present-Day Hospital Readmissions after Left Ventricular Assist Device Implantation: A Large Single-Center Study. Tex Heart Inst J 2015;42:419-29. [Crossref] [PubMed]
- Kimura M, Nawata K, Kinoshita O, et al. Readmissions after continuous flow left ventricular assist device implantation. J Artif Organs 2017;20:311-7. [Crossref] [PubMed]
- Smedira NG, Hoercher KJ, Lima B, et al. Unplanned hospital readmissions after HeartMate II implantation: frequency, risk factors, and impact on resource use and survival. JACC Heart Fail 2013;1:31-9. [Crossref] [PubMed]
- Jakovljevic DG, McDiarmid A, Hallsworth K, et al. Effect of left ventricular assist device implantation and heart transplantation on habitual physical activity and quality of life. Am J Cardiol 2014;114:88-93. [Crossref] [PubMed]
- Casida JM, Wu HS, Abshire M, et al. Cognition and adherence are self-management factors predicting the quality of life of adults living with a left ventricular assist device. J Heart Lung Transplant 2017;36:325-30. [Crossref] [PubMed]
- Celler B, Varnfield M, Nepal S, et al. Impact of At-Home Telemonitoring on Health Services Expenditure and Hospital Admissions in Patients With Chronic Conditions: Before and After Control Intervention Analysis. JMIR Med Inform 2017;5:e29. [Crossref] [PubMed]
- Chaudhry SI, Phillips CO, Stewart SS, et al. Telemonitoring for patients with chronic heart failure: a systematic review. J Card Fail 2007;13:56-62. [Crossref] [PubMed]
- Koehler F, Winkler S, Schieber M, et al. Telemedical Interventional Monitoring in Heart Failure (TIM-HF), a randomized, controlled intervention trial investigating the impact of telemedicine on mortality in ambulatory patients with heart failure: study design. Eur J Heart Fail 2010;12:1354-62. [Crossref] [PubMed]
- Koehler F, Winkler S, Schieber M, et al. Telemedicine in heart failure: pre-specified and exploratory subgroup analyses from the TIM-HF trial. Int J Cardiol 2012;161:143-50. [Crossref] [PubMed]
- Pektok E, Demirozu ZT, Arat N, et al. Remote monitoring of left ventricular assist device parameters after HeartAssist-5 implantation. Artif Organs 2013;37:820-5. [PubMed]
- Lampert BC, Emani S. Remote hemodynamic monitoring for ambulatory left ventricular assist device patients. J Thorac Dis 2015;7:2165-71. [PubMed]
- Eifert S, Meyer A, Lehmann S, et al. Anticoagulative Treatment in LVAD Patients: Telemonitoring by Use of Vitaphone®. Thorac cardiovasc Surg 2016.64.
- Guglin M, George B, Branam S, et al. CardioMEMS™ in LVAD Patients: A Case Series. 2016. Available online: http://uknowledge.uky.edu/cgi/viewcontent.cgi?article=1056&context=vad
- Duquenoy P, Mekawie MN, Springett M. Patients, trust and ethics in information privacy in eHealth. In: George C, Whitehouse D, Duquenoy P (Hrsg.). editors. eHealth: Legal, ethical and governance challenges. Heidelberg: Springer, 2013:275-98.
- Fisk MJ, Rudel D. Telehealth and service delivery in the home - care, support and the importance of user autonomy. In: George C, Whitehouse D, Duquenoy P (Hrsg.). editors. eHealth: Legal, ethical and governance challenges. Heidelberg: Springer, 2013:211-26.
- Ionescu-Dima C. Legal challenges regarding telemedicine services in the European Union. In: George C, Whitehouse D, Duquenoy P (Hrsg.). editors. eHealth: Legal, ethical and governance challenges. Heidelberg: Springer, 2013:107-34.
- Critoph C, Green G, Hayes H, et al. Clinical Outcomes of Patients Treated With Pulmonary Vasodilators Early and in High Dose After Left Ventricular Assist Device Implantation. Artif Organs 2016;40:106-14. [Crossref] [PubMed]
- Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658-66. [Crossref] [PubMed]
- Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:935-44. [Crossref] [PubMed]
- Wasson LT, Yuzefpolskaya M, Wakabayashi M, et al. Hypertension: an unstudied potential risk factor for adverse outcomes during continuous flow ventricular assist device support. Heart Fail Rev 2015;20:317-22. [Crossref] [PubMed]
- Hindricks G, Taborsky M, Glikson M, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet 2014;384:583-90. [Crossref] [PubMed]
- Vollmann D, Nägele H, Schauerte P, et al. Clinical utility of intrathoracic impedance monitoring to alert patients with an implanted device of deteriorating chronic heart failure. Eur Heart J 2007;28:1835-40. [Crossref] [PubMed]
- Bartoli CR, Vessels KM, McCants KC. Increased intrathoracic impedance may predict adverse events in LVAD patients. J Card Surg 2013;28:616-8. [Crossref] [PubMed]
- Dionizovik-Dimanovski M, Levin AP, Fried J, et al. Correlation Between Home INR and Core Laboratory INR in Patients Supported with Continuous-Flow Left Ventricular Assist Devices. ASAIO J 2015;61:386-90. [Crossref] [PubMed]
- Koertke H, Zittermann A, Wagner O, et al. Self-management of oral anticoagulation therapy improves long-term survival in patients with mechanical heart valve replacement. Ann Thorac Surg 2007;83:24-9. [Crossref] [PubMed]
- Zierer A, Melby SJ, Voeller RK, et al. Late-onset driveline infections: the Achilles' heel of prolonged left ventricular assist device support. Ann Thorac Surg 2007;84:515-20. [Crossref] [PubMed]
- Pieri M, Scandroglio AM, Müller M, et al. Surgical management of driveline infections in patients with left ventricular assist devices. J Card Surg 2016;31:765-71. [Crossref] [PubMed]
- Reiss N, Schmidt T, Müller-von Aschwege F, et al. Telemonitoring and Medical Care of Heart Failure Patients Supported by Left Ventricular Assist Devices - The Medolution Project. Stud Health Technol Inform 2017;236:267-74. [PubMed]
- Granegger M, Schlöglhofer T, Ober H, et al. Daily life activity in patients with left ventricular assist devices. Int J Artif Organs 2016;39:22-7. [Crossref] [PubMed]
- Hellman Y, Malik AS, Lane KA, et al. Pulse Oximeter Derived Blood Pressure Measurement in Patients With a Continuous Flow Left Ventricular Assist Device. Artif Organs 2017;41:424-30. [Crossref] [PubMed]


