A population-based analysis of outcomes after radiotherapy in intensive care unit patients with lung cancer
Introduction
Lung cancer accounts for approximately 25% of all solid malignancy intensive care unit (ICU) admissions (1). The reasons for ICU care in this patient population most commonly relate to malignant sequelae (2), which in some instances may necessitate urgent anticancer treatment. Overall, prognosis in these patients is dismal, as approximately 1 in 4 will die during their hospital stay, with fewer than 50% surviving beyond 6 months (3).
Although ICU admission for life-threatening events in lung cancer patients is viewed by some as futile, an increasing number of single institution (4-6) and population-based (3,7) studies evaluating both prognostic factors and survival outcomes have been helpful in guiding individual patient decision making. In these studies, however, the use of radiotherapy (RT) has not been systematically assessed.
Although RT is a cornerstone in the palliative management of lung cancer (8), its use for patients in the ICU is poorly described. To our knowledge, a single study of 26 patients, evaluating the extreme scenario of RT for malignant airway obstruction necessitating mechanical ventilation (9,10), represents the only literature focusing on the use of RT for intrathoracic malignancy in the ICU.
As the delivery of RT is an involved and costly process that may merely prolong ICU stays and/or delay a proper transition to end-of-life care planning, further examination of such patients is warranted. Thus, the purpose of this study is to describe characteristics, outcomes and RT use, in a population-based cohort of lung cancer patients admitted to the ICU in the province of Ontario.
Methods
Study design and cohort selection
This is a population-based, retrospective study of a cohort of lung cancer patients admitted to the ICU in Ontario, Canada, between April 1, 2007, and March 31, 2014. Patients were excluded if (I) they did not have a valid provincial health insurance number; (II) they were not an Ontario resident; or (III) if they were younger than 18 years or older than 105 years at the time of diagnosis. The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board. This project was approved through a retrospective review process by the institutional review board at Sunnybrook Health Sciences Centre, Toronto, Canada.
Data sources
Multiple administrative health care databases were used to build the cohort of interest. These datasets were linked using unique encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences (ICES). Records of ICU hospitalization were identified using the Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD) (11). RT and ICU ventilation codes were obtained from the same database, as it captures all diagnostics and procedures from all inpatient hospital admissions. To verify cancer diagnosis, we used the Ontario Cancer Registry (OCR), which contains baseline information on approximately 98% of incident malignancies in a population of 14 million (12). In Ontario, physician services provided to patients are covered through a universal health plan, thus all billings related to ICU care, Intensivist/Oncologist consultation and RT administration were obtained from the Ontario Health Insurance Plan (OHIP) claims database. Finally, using the Registered Person’s Database we obtained basic patient demographic information, such as socioeconomic data and vital statistics.
Patient factors
As patients could be eligible for multiple ICU admissions, each consecutive-day admission into a facility designated as an ICU was defined as an episode of care. Differences in the receipt of RT were examined across patient age, sex, income and region of residence. Patients were assigned rural residence status if the population size of their community was 10,000 or less. City postal codes were used to derive Local Health Integration Network (LHIN) designation and rural residence. In Ontario, healthcare delivery is regionalized through 14 LHINs, and each LHIN represents a group of facilities and services responsible for the provision of healthcare to a population within a defined geographic area. Patient comorbidity was classified using the Charlson Index modified for administrative data (13), with a lookback period of 5 years from the index ICU admission date. International Classification of Disease tenth revision (ICD-10) codes were used to identify cancer type (i.e., primary lung versus metastatic neoplasm). Patients were evaluated for previous health care system use, including treatment with RT and ICU admission.
Institution and treatment information
As the delivery of RT for cancer in Ontario is centralized to regional cancer centers, to receive RT, ICU patients could be transported within a hospital that provides both ICU and RT services, or transferred from a peripheral ICU unit to a hospital that delivers RT. The total number of transfers between institutions and total number of ICU visits were counted for all RT and non-RT patients. The disposition before ICU stay was abstracted, stratified by admission to ICU from the emergency room (ER) and transferred from a different institution or admission within the same institution. Disposition after ICU stay was also obtained and included death within ICU, transfer to a different institution and discharge within the same institution. Finally, criteria for defining mechanical ventilation vary between OHIP and CIHI (14), and should be considered when classifying patients according to this procedure. In combination, these codes have been demonstrated to be accurate in identifying ICU admission as well as the use of some form of ventilation, however, their reliability in defining invasive mechanical ventilation is less known.
Statistical analysis
Significant differences between RT and non-RT groups were compared using two sample t-tests for continuous variables and Chi-square test for categorical variables. Additionally, standardized differences were obtained for all comparisons, with values >0.10 indicative of imbalance between groups (15,16). The Kaplan-Meier method was used to estimate overall survival (OS), measured from the time of index ICU admission to death, with censorship at last follow-up, up to a maximum of 1 year. Differences in OS between the RT and non-RT groups were compared using the log-rank test. Univariable and multivariable Cox proportional hazards modeling were performed to assess the effect of RT on survival. The multivariable Cox model was built through manual selection of variables based on clinical knowledge and statistical properties, including: age, sex, rural status, income quintile, Charlson comorbidity index, LHIN, previous treatment, previous ICU stay, ventilation status, and histology. The proportional hazard assumption was assessed in building the final multivariable model. All statistical analyses were performed using SAS version 9.4 (SAS institute, Cary NC, USA) using two-sided testing with a p-value threshold of <0.05 employed as statistical significance.
Results
As depicted in Figure 1, in the 13,739 unique ICU patients with lung cancer meeting the inclusion criteria, there were a total of 14,710 episodes of care. Within these, episodes of RT delivery were rare (n=133, 0.9%; across n=131 patients). The RT group tended to be younger (median age 65 vs. 68, P<0.001), but with worse baseline characteristics, including being on some form of supportive ventilation in the ICU (79.8% vs. 38.2%, P<0.001), and longer ventilation duration {median (IQR): 6 [1–11] vs. 0 [0–2] days, P<0.001}. Pre-ICU disposition in RT patients was more likely to be from the ER (28.6% vs. 21.9%) or via transfer from a different institution (35.3% vs. 9.7%) (P<0.001). The RT group tended to have increased healthcare utilization, with more frequent prior ICU stays (mean: 1.53 vs. 1.37, P=0.029). Additional characteristics, stratified by the receipt of RT, are summarized in Tables 1-3.
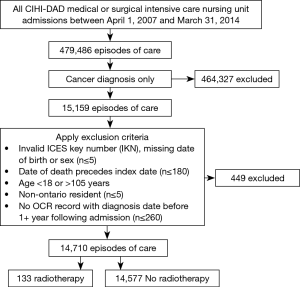
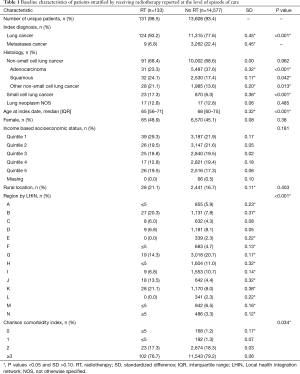
Full table
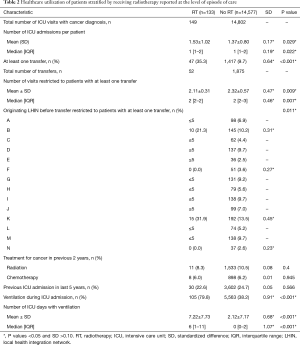
Full table

Full table
RT delivery varied across geographic LHIN regions, with half of the 14 LHINs treating 5 patients or fewer. Similarly, 2 LHINs accounted for the majority of patients who were transferred from one institution to another institution for ICU admission and RT treatment (21.3% and 31.9% of total RT patients, respectively). In contrast, in the non-RT group, institutional transfer was less common, ranging from 2.6% to 13.5% across LHINs. RT details are summarized in Table 4.
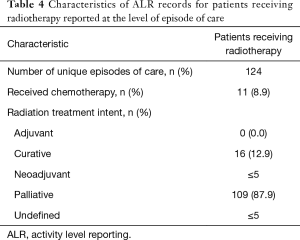
Full table
Of the non-RT patients, 536 (3.7%) were identified as having received RT to a non-thoracic target. In this non-RT cohort, most (84.7%) did not have a consultation with a Radiation Oncologist within 1 month prior to ICU admission. In contrast, for RT patients, most Radiation Oncologist consultations were within the ICU admission episode (78.9%).
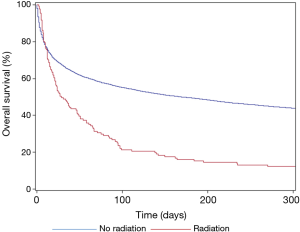
Post-ICU disposition differed between the two cohorts, with RT patients more commonly dying in the ICU (27.1% vs. 17.6%), and less frequently being discharged into the same institution (49.6% vs. 67.2%) (P<0.001). While ICU discharge was common in both RT (n=75, 56.4%) and non-RT (n=10,405, 71.4%) cohorts, 1-year OS was poor with both groups, but most notably in the RT group (11.3% vs. 42.4%). OS, stratified by the use of RT, is shown in Figure 2. RT was associated with inferior 1-year OS on unadjusted modeling (HR: 1.99, 95% CI: 1.65–2.38, P<0.001), with ventilation status and pre-ICU disposition adjusting this finding towards the null on multivariable modeling (HR: 1.17, 95% CI: 0.97–1.40, P=0.095).
Discussion
Although literature on management and outcomes of patients with lung cancer admitted to the ICU is growing, across studies, there remains a paucity of RT-specific data. The value of RT in these patients is of uncertain efficacy; yet RT may be associated with prolongation of ICU stays, delay of transition to end-of-life care, and increased costs. Given the increasing rates of ICU care for lung cancer patients worldwide and the proven role of RT in lung cancer in general, we report a population-based analysis, to our knowledge the largest such study, focusing on patterns of RT treatment, outcomes, and potential predictors of survival. There are several key findings in the present study. First, although ICU admission in lung cancer patients is associated with a poor OS, a significant minority of patients treated with RT may be discharged from the ICU and achieve prolonged survival, suggesting that the use of RT may not be futile. Second, despite the centralized delivery of RT in larger centers within Ontario, there are significant disparities on the delivery of RT, whereby the majority of RT treatments occur in one of a few centers. This result is highlighted by the differential willingness to refer and/or consider RT within regions of practice, as reflected by the low rate of radiation oncology consultation (15.3%) in the non-RT group. Third, RT patients have higher rates of transfer from the ER or another institution (rather than from within the same institution) to the ICU, as well as higher rates of ventilation when compared to their non-RT counterparts. When adjusting for these two negative prognostic factors on multivariable regression modeling, there was no difference in OS between the two groups, again suggesting that the use of RT may not be futile.
The present population study builds on the current knowledge regarding the management of lung cancer patients in the ICU. In a multi-institutional prospective study of 449 lung cancer patients in the ICU, it was reported that approximately 50% had cancer-related complications, in which a minority of patients were treated emergently with chemotherapy (n=25, 5%) or radiation (n=5, 1%). Most admissions were because of a new diagnosis of lung cancer (n=318, 71%), with airway compromise by tumor (n=116, 26%) being the most commonly cited reason.
Our institutional experience on the use of RT for malignant airway obstruction necessitating mechanical ventilation in the ICU suggested that RT resulted in extubation success (ES, defined as ≥48 hours of extubation without re-intubation) in 27% of patients. Recognizing the small number of patients in this retrospective review, the use of higher doses of RT appeared to be associated with both ES and OS on regression analyses. In the present population study, RT doses and more granular information related to the type of invasive ventilation used were not routinely available, which limits the ability to support our previous hypothesis-generating conclusions.
Other anticancer therapies have been evaluated for the ICU lung cancer patient. In small cell lung cancers, platinum-based doublet chemotherapies have been reported to result in dramatic responses in selected case series (17,18). Regarding newer targeted agents, Inoue and colleagues performed a phase II study investigating the efficacy and feasibility of gefitinib in advanced NSCLC patients harboring epidermal growth factor receptor (EGFR) mutations (19). In this small cohort of predominantly poor [3–4] performance status (PS) patients, the overall response rate was 66%. In fact, 68% of the PS 3–4 group had improvement in their PS to ≤1 after treatment. The literature on this so-called Lazarus response on lung cancer patients with oncogenic mutations (i.e., EGFR and anaplastic lymphoma kinase, ALK) in the ICU was recently reviewed, with the identification of 6 case reports and an additional 4 articles describing institutional outcomes (20). In select cases, empiric EGFR tyrosine kinase inhibitor was administered, on the basis of risk factors such as non-smoker, female and Asian race. Although there are growing reports arguing for the suitability of targeted agents for critically ill lung cancer patients in the ICU, to our knowledge, there are no reports describing such outcomes with newer immunotherapy agents. Indeed, in selected patients immunotherapy has compared favorably with standard first-line chemotherapy in the metastatic non-small cell lung cancer setting (21), however; this potentially paradigm-shifting development may also have important ramifications for the ICU clinician. As Guillon et al. state, “we have entered a time when triage criteria based on lung cancer prognosis will be almost impossible to define for the ICU clinician. Consequently, ICU admissions will have to be determined by high-quality consultation between the intensivist and thoracic-oncologist to define prognosis and appropriate treatment goals” (22).
As is typically the case when using administrative data, the results of the present study need to be considered in the context of inherent limitations due to lack of information regarding relevant clinical characteristics such as the goals of care, PS, and true mechanical ventilation status. Given the poor overall health of the cohort studied, it is difficult to fully understand the complex interaction of lung cancer, competing risk and the potential effect of RT.
Conclusions
It is clear that lung cancer patients in the ICU within Ontario have a poor OS. The use of RT in this setting is rare, and geographic disparities exist in its utilization within a publically funded healthcare system. Those receiving RT are more likely to present via transfer or the ER, with a significant proportion achieving discharge and a minority prolonged survival, suggesting that RT use may not be futile. Thus, further research is warranted to elucidate the role of RT in these patients, to look at strategies to mitigate challenges in its utilization and to determine which patients may incur the most benefit from its use.
Acknowledgements
This work was supported by the Institute for Clinical Evaluative Sciences (ICES) Western site. ICES is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Core funding for ICES Western is provided by the Academic Medical Organization of Southwestern Ontario (AMOSO), the Schulich School of Medicine and Dentistry (SSMD), Western University, and the Lawson Health Research Institute (LHRI). Parts of this material are based on the data and information compiled and provided by CIHI and Cancer Care Ontario (CCO). However, the analyses conclusions, opinions and statements expressed herein are those of the author, and not necessarily those of CIHI or CCO. The opinions, results and conclusions are those of the authors and are independent from the funding sources. No endorsement by ICES, AMOSO, SSMD, LHRI or the MOHLTC is intended or should be inferred. Dr. Louie’s research is funded by the Schulich Clinician Scientist Award. He has received speaker’s honoraria from Varian Medical Systems Inc.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board. This project was approved through a retrospective review process by the institutional review board at Sunnybrook Health Sciences Centre, Toronto, Canada.
References
- Soares M, Caruso P, Silva E, et al. Characteristics and outcomes of patients with cancer requiring admission to intensive care units: a prospective multicenter study. Crit Care Med 2010;38:9-15. [Crossref] [PubMed]
- Cooke CR, Feemster LC, Wiener RS, et al. Aggressiveness of intensive care use among patients with lung cancer in the Surveillance, Epidemiology, and End Results-Medicare registry. Chest 2014;146:916-23. [Crossref] [PubMed]
- Slatore CG, Cecere LM, Letourneau JL, et al. Intensive care unit outcomes among patients with lung cancer in the surveillance, epidemiology, and end results-medicare registry. J Clin Oncol 2012;30:1686-91. [Crossref] [PubMed]
- Lin YC, Tsai YH, Huang CC, et al. Outcome of lung cancer patients with acute respiratory failure requiring mechanical ventilation. Respir Med 2004;98:43-51. [Crossref] [PubMed]
- Andréjak C, Terzi N, Thielen S, et al. Admission of advanced lung cancer patients to intensive care unit: a retrospective study of 76 patients. BMC Cancer 2011;11:159. [Crossref] [PubMed]
- Roques S, Parrot A, Lavole A, et al. Six-month prognosis of patients with lung cancer admitted to the intensive care unit. Intensive Care Med 2009;35:2044-50. [Crossref] [PubMed]
- Bonomi MR, Smith CB, Mhango G, et al. Outcomes of elderly patients with stage IIIB-IV non-small cell lung cancer admitted to the intensive care unit. Lung Cancer 2012;77:600-4. [Crossref] [PubMed]
- Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol 2011;1:60-71. [Crossref] [PubMed]
- Louie AV, Lane S, Palma DA, et al. Radiotherapy for intubated patients with malignant airway obstruction: futile or facilitating extubation? J Thorac Oncol 2013;8:1365-70. [Crossref] [PubMed]
- Louie AV, Lane S, Rodrigues GB. Future directions in palliative radiotherapy for malignant airway obstruction requiring mechanical ventilation. J Thorac Oncol 2014;9:e54-5. [Crossref] [PubMed]
- Canadian Institute for Health Information: Data Quality Documentation, Discharge Abstract Database. 2009-2010: Executive Summary. 2010. Available online: https://www.cihi.ca/en/dad_executive_sum_09_10_en.pdf
- Robles SC, Marrett LD, Clarke EA, et al. An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol 1988;41:495-501. [Crossref] [PubMed]
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9. [Crossref] [PubMed]
- Scales DC, Guan J, Martin CM, et al. Administrative data accurately identified intensive care unit admissions in Ontario. J Clin Epidemiol 2006;59:802-7. [Crossref] [PubMed]
- Austin PC, Mamdani MM. A comparison of propensity score methods: a case-study estimating the effectiveness of post-AMI statin use. Stat Med 2006;25:2084-106. [Crossref] [PubMed]
- Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 2007;26:734-53. [Crossref] [PubMed]
- Jennens RR, Rosenthal MA, Mitchell P, et al. Outcome of patients admitted to the intensive care unit with newly diagnosed small cell lung cancer. Lung Cancer 2002;38:291-6. [Crossref] [PubMed]
- Song JU, Suh GY, Chung MP, et al. Risk factors to predict outcome in critically ill cancer patients receiving chemotherapy in the intensive care unit. Support Care Cancer 2011;19:491-5. [Crossref] [PubMed]
- Inoue A, Kobayashi K, Usui K, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol 2009;27:1394-400. [Crossref] [PubMed]
- Langer CJ. The "lazarus response" in treatment-naive, poor performance status patients with non-small-cell lung cancer and epidermal growth factor receptor mutation. J Clin Oncol 2009;27:1350-4. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Guillon A, Reckamp KL, Heuzé-Vourc'h N. Immunotherapy improves the prognosis of lung cancer: do we have to change intensive care unit admission and triage guidelines? Crit Care 2017;21:18. [Crossref] [PubMed]


