Single-port video-assisted thoracoscopic surgery for lung cancer
A new trend: video-assisted thoracoscopic surgery (VATS) for lung cancer
On October 19, 2013, four live demonstrations of successful single-port VATS lobectomies were performed at Shanghai Pulmonary Hospital. More than 200 chest surgeons witnessed this demonstration and shared their thoughts. Based on this response, it is clear that lung cancer, and, in particular, single-port VATS lobectomy for lung cancer, are popular topics among Asian thoracic surgeons (1,2).
Both the general public and the government are aware of the high incidence, morbidity, and mortality rates (as well as the high medical costs) associated with advanced-stage lung cancer. Several screening tools, including chest radiography and low dose computed tomography (CT) (3,4), have been advocated in high risk patients. As a result, more patients are presenting with early-stage lung cancer. If they can be adequately treated, they usually harbor a better prognostic outcome. These patients are also, potentially, the best candidates for minimally invasive surgery which can reduce their recovery time and suffering after surgery. Thus, we will focus on the role of single-port VATS for lung cancer lobectomy.
Current VATS practice in Taiwan
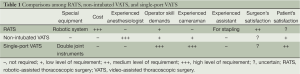
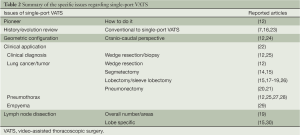
Before 2000, conventional posterolateral open thoracotomy remained the “gold standard” for the treatment of patients with lung cancer. However, VATS has replaced open thoracotomy and has become the current mainstay of lung cancer surgery (5). Actually, the penetration rate of VATS in Taiwan is extremely high. As surgical creativity and innovation have progressed, three variants of VATS have been developed in our society, the robotic-assisted thoracoscopic surgery (RATS), non-intubated VATS, and single-port VATS (6,7). Differences regarding cost, instrument settings, special anesthesia demands, surgical techniques, patient selection, and insurance coverage among these VATS alternatives are quite difficult to have evidence-based comparison (Table 1). Shows the summary based on our interpretation.
Full table
For lung cancer surgery, several controversies exist concerning RATS (8), including the high cost, the increased number of utility wounds, and the need for skilled assistants to perform stapling for bronchus and pulmonary vessels. However, with the advances in new instrumentation, especially the endocutter, and the reduced size of the robotic arm which has avoided collisions during surgery, the role of RATS in lung cancer has exceeded our expectations. Although non-intubated VATS did not represent an improvement in surgical technique, it has the advantage of reduction in airway trauma caused by standard double-lumen endotracheal intubation. Furthermore, both the introduction of vagus nerve blockade to inhibit cough reflex and the insertion of an epidural catheter to reduce pain emphasize the need for team work between chest surgeon and anesthesiologist (9,10) during this technique.
Due to high cost limitations associated with RATS or the discomfort associated with mediastinal movement during non-intubated VATS, single-port VATS is another option for chest surgeons who are familiar with the conventional 4-, 3- or 2-ports VATS. In this review, we will assess the various uses of single-port VATS, especially for lobectomy.
Current evidence
Single-port surgery has been adopted in several surgical fields, especially in colorectal and gynecologic training programs (11). In 2004, Rocco et al. reported their pioneering work with pulmonary wedge resection through single-port VATS (12). Thereafter, more and more chest surgeons used single-port VATS for pulmonary resection, including wedge resection (12), segmentectomy (13-15), lobectomy (16-19), pneumonectomy (20,21), and pleural surgery (including pleural biopsy and pleural resection or decortication) (22), for both benign or malignant disease (Table 2). Lobectomy plus radical mediastinal lymph node dissection remain the “gold standard” for resectable lung cancer. However, pneumonectomy or segmentectomy/wedge resection may be executed based on lung cancer status, according to oncological principles or clinical considerations. Between 2012 and 2013, Gonzalez-Rivas et al. shared their innovative experiences with single-port VATS lobectomy, segmentectomy, and pneumonectomy for lung cancer (13,14,17-21). They also explained, in detail, the procedure and necessary equipment for meticulous application of single-port VATS. However, this was only one report from a single institution. The advantages and disadvantages of single-port VATS vs. conventional 2-, 3-,or 4-ports VATS, especially for lobectomy or segmentectomy of lung cancer, deserved further re-appraisal.
Full table
Our experiences at the Koo Foundation Sun Yat-Sen Cancer Center
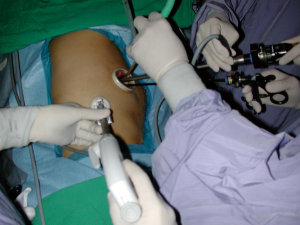
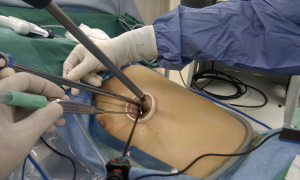
We began performing 3-ports VATS lobectomy in 2005, and shifted to 2-ports VATS lobectomy in 2007. As described by Rocco et al. (12), surgeons always stand in front of the patient while performing VATS, in contrast to conventional open thoracotomy. Concerning the 2-ports VATS lobectomy that we perform, one port is used for instrument insertion (utility port, 3-5 cm in length, retracted by wound protector) and the other port is used for camera scope insertion (scope port, 1 cm in length, kept by trocar). A 30-degree camera scope was applied to our VATS. Actually, during 2-ports lobectomy, we insert multiple instruments into the utility port, which is the training basis for single-port VATS lobectomy. During upper lobe lobectomy, the straight endocutter is usually shifted to the camera port for the division of superior pulmonary vein, and all other instruments plus thoracoscope are inserted through the utility port (Figure 1). More specifically, if we can insert the endocutter through the same wound, i.e., the utility port, it becomes a single-port VATS. The application of the curved endocutter plays an important role in this specific procedure (Figure 2).
We began single-port VATS lobectomy in December 2010 (30). The first case we performed was actually a single-port segmentectomy for a centrally located carcinoid tumor over the left common basilar segment of the left lower lung. Because this patient had chronic obstructive pulmonary disease (COPD) with poor lung function, we shifted our tentative single-port VATS lobectomy to a segmentectomy, and this may represent the first single-port VATS segmentectomy reported in the literature (15,30). Despite the severely adherent anthracotic segmental lymph nodes, we completed the procedure smoothly and successfully. Since that time, we have been capable of single-port VATS anatomic lung resection. However, during that period, the concept of single-port VATS lobectomy or segmentectomy was neither popular nor well accepted. Most chest surgeons performed VATS lobectomy through 2-, 3-, or 4-ports procedures, or through a needle scope. Inevitably, however, a small utility wound is necessary for surgical specimen retrieval. Based on the need to reduce surgical trauma, we began our single-port VATS lobectomy/segmentectomy program, depending on careful case selection, the need for resident training, or sufficient surgical time without a tight schedule. Between November 2010 and May 2012, we retrospectively collected 19 cases of single-port VATS lobectomy/segmentectomy at our institute, and we shared our experiences with an emphasis on patient safety and surgical skill during radical lymph node dissection. This preliminary experience demonstrated the feasibility of single-port VATS lobectomy and radical lymph node dissection for benign pulmonary disease and early-stage lung cancer (15).
In Dr. C.C. Liu’s practice, single-port lobectomy has become the standard procedure for lung cancer surgery, if there is no chest wall invasion or obvious hilar structural invasion. The size of the tumor is seldom a contraindication, since the larger the tumor, the larger the utility wound needed. Usually, the incision is approximately 3 cm in length if the tumor diameter is less than 3 cm.
Our collection of cases totals 63 single-port VATS lobectomies and 24 segmentectomies. The conversion rate from single-port VATS was low (3.45%, 3 in 87, converted to 2-ports VATS because of adhesion/anthracotic lymph nodes) and no surgical mortality occurred. Post-operative recovery was similar to traditional 2-ports VATS lobectomies.
The concept of minimally invasive surgery is not only preferred for reduction in the size of the external wound but also for reduction in inner trauma, including the extent of tumor resection and lymph node dissection. Therefore, sub-lobar resection (especially segmentectomy) for early stage lung cancer is crucial (31). Regarding the resection planes used in segmentectomy, we prefer the endocutter stapler to seal off air leakage rather than electrical coagulation (32). The segmental structures, including segmental artery, bronchus and veins, are dissected towards the hilum and then divided. The dissected lymph nodes and the resection margins deserve special mention. They are examined by experienced pathologists through frozen section to guarantee a complete resection. One of our patients harbored a ground glass opacity (GGO) lesion over the basal segment of the lower lobe, which proved to be lung cancer during intra-operative pathological diagnosis. The basal segmentectomy was converted to lobectomy due to a close resection margin. At present, we have already performed 24 segmentectomies using endocutter stapler to divide the intersegmental planes. Compared with conventional cauterization along the intersegmental plane and for ligation of branches from intersegmental veins, endocutter stapler is much easier to use. Although transient postoperative lung atelectasis with subsequent fever is not inevitable, this procedure does reduce the incidence of prolonged or delayed air leak (33). With regards to conventional segmentectomies, e.g., superior segmentectomy for the lower lobe, lingular segmentectomy, trisegmentectomy of left upper lobe (lingular sparing) and common basal segmentectomy of lower lobe, they can all be executed using a single-port technique. We have also begun lobe-specific lymph node dissection for early stage lung cancer. It not only reduces the degree of mediastinal trauma and saves more time, but also lessens the complications related to extensive lymph node dissection (15).
OR setting
We use the same setting as used in traditional VATS in our group.
Anesthesia
General anesthesia with double lumen endotracheal tube intubation is applied for right sided procedures. For left sided procedures, in contrast, single lumen endotracheal tube intubation with endobronchial blocker is applied, especially when left subcarinal lymph node dissection is required. A low tidal volume of approximately 350 mL with a PEEP at a setting of approximately 5 mmHg is preferred. Central venous catheter insertion is not our routine. Intercostal nerve block with bupivacaine is injected by the surgeon along the utility wound [including one more intercostal space (ICS) up and down] at the end of surgery.
Patient positioning
We use the same position as used in traditional VATS.
Instruments
Scanlan® VATS instruments, laparoscopic grasp, laparoscopic needle holder, knot pusher, and harmonic scalpel are the main instruments used during single-port VATS. We prefer to use longer instruments to avoid their possible collision due to crowding during manipulation. The surgical field can be viewed clearly through a 10 mm (preferred) or 5 mm 30 degree endoscope. The utility wound is retracted by wound protector (XS in size).
Incision
We prefer to create the utility wound at the sixth ICS that crosses the anterior axillary line for the following reasons:
- For upper lobe lobectomy, this incision is away from the superior pulmonary vein and provides adequate space for applying the endocutter;
- For lymph node dissection, this incision allows for easier subcarinal lymph node dissection, especially when we encounter a bulky bronchial tree caused by double lumen tube with an inflated balloon or a relatively rigid bronchus;
- Such an incision avoids hypersensitive areas or avoids causing paresthesia involving the breast, particularly the nipple, because the nipple is innervated by 4th intercostal nerve;
- The incision may be shifted more laterally along the sixth ICS in female patients, and along the fifth ICS only for left upper lobe LB1+2,3 trisegmentectomy for early lung cancer and lobe specific lymph node dissection and the upper mediastinum lymph nodes, rather than the subcarinal lymph nodes.
Handling of instruments
To achieve a smooth surgical procedure, the long curved sucker (Scanlan®) is manipulated by the surgeon’s left hand, and the hook, laparoscopic or Scanlan® dissectors are manipulated using his right hand. Such a combination of long straight and long curved instruments can minimize their collision and interference during surgery. Generally, the entire single-port VATS procedure is similar to traditional 2-ports VATS, except for the application of the endocutter through a narrower space that requires more skill. Adequate dissection and release of the surrounding soft tissues of the vascular structures are important steps that provide sufficient space for insertion of the endocutter blade during conventional 3- or 2-ports VATS. The Endo-GIA was originally designed for gastrointestinal anastomosis, rather than pulmonary vascular structures. We feel that it is a problem of instrument design rather than a problem with technique. The newly designed curved-tip endocutter is a useful option when performing division of a vessel, especially the superior pulmonary vein.
Camera scope handling
An experienced cameraman is crucial for a successful single-port VATS lobectomy, and a 30-degree camera scope (10 or 5 mm) is recommended. After an initial inspection of the surgical field from the eagle view through the camera, an impression of the anatomic landmarks should be fully realized. The surgeon can re-adjust the settings to facilitate the exposure and dissection. Usually the surgeon will choose the best position for performing the surgical procedure, and then bring in the camera to take advantage of the 30-degree lens to provide the best view. Every step should be under direct vision in order maintain safety, especially during dissection and application of the endocutter to the great vessels. Concerning the relative positions inside the utility port, grasp and camera scope are usually maintained in the upper part of the port and the surgeon’s instruments are in the lower part during dissecting process. However, during application of the endocutter or other specific procedures, a dynamic change in position may be necessary in order to command a clearer view and perform a safer procedure (Figure 2).
VATS lymph node dissection
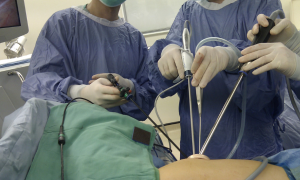
Lymph node dissections on the right side of the mediastinum and left upper mediastinum are similar to traditional 2-ports VATS procedure (Figures 3,4). Although usually not difficult, it requires more time and patience to perform. However, the left subcarinal area can be challenging. We developed a special method, called the Liu’s maneuver, to facilitate exposure of the left subcarinal space. We placed a non-elastic bandage above the inferior pulmonary vein to hook on the left lower lobe bronchus; thus, the lung parenchyma and hilum can be pulled away from the aorta and esophagus. With this maneuver, we obtain a clearer view of the carina, bilateral main bronchi, bilateral inferior pulmonary veins, pericardium, and esophagus along with right and left vagus nerves, right lung and right mediastinal pleura. In our early series using this maneuver, the average number of dissected lymph nodes for lung cancer was 23, similar to traditional VATS (15).
Learning curve, education, and training
Regarding learning curves, we went through a process similar to that of Diego Gonzalez-Rivas (6,16). As shown in his review articles, he also shifted from 3-ports to 2-ports and then to single-port VATS lobectomy. As a result, this process could be a training model for those who had VATS experience and who wanted to shift to single-port VATS lobectomy, and for the new learner starting VATS lobectomy.
However, for the new learner without VATS experience, we are not certain about the need for a shift from 3-ports to 2-ports and then to single-port VATS. This question is similar to questions regarding the training required for traditional VATS. An open procedure is not absolutely necessary for the trainee to learn VATS. Similar to Diego Gonzalez-Rivas’s group, we found that trainees without previous VATS experience are more open to new ideas and settings using single-port VATS. Actually, they accept these procedures and perform them better and more easily than experienced VATS surgeons who have already performed multiple ports VATS.
There is insufficient supporting data concerning the learning curve associated with VATS. Some thought that experienced VATS surgeons harbored a basic concept for VATS, and thus they could reach the plateau of the learning curve much quicker. However, if we take the Cases/Time curve into consideration when predicting the learning curve, perhaps the new learner will reach the learning plateau quicker than I did as it took me two years to pass the learning curve by accumulating more than 30 cases, which was really slow going in the beginning! This was not due to the difficulty of the technique, but rather the difficulty in making the determination whether or not to do it. To quote a well-known proverb:
The Difficulty lies, not in the new ideas, but in escaping from the old ones
——By John Maynard Keynes
The aggressive mind of the chest surgeon plays an important role in promoting the rapid development of commercial equipment necessary for single-port VATS, including the single-port wound protector, articulating instruments, harmonic scalpel, various sized straight or curved endocutter staplers, and higher resolution camera scopes with less scopic diameter. The mutual interaction between the desire of the surgeon and new manufacturing designs is the stimulus for advances in VATS.
More and more single-port VATS symposiums and conferences are held worldwide. Specific training programs on single-port VATS are also available. Furthermore, there are many single-port VATS videos, including trouble shooting for incomplete fissures, anthracotic lymph nodes, sleeve resection/bronchoplasty, bleeding, etc. These videos are available over the internet through YouTube.
Careful patient selection by the chest surgeon, for either benign or malignant disease, plays an important role when starting a single-port VATS lobectomy program. A single port VATS lower lobe lobectomy can be accomplished as easily as traditional VATS if there are no anthracotic nodes in the hilum nor incomplete fissures. The chest surgeon can then gradually expand his expertise to include all patients with early-stage lung cancer, if there are no specific contraindications.
In conclusion, we have reviewed the feasibility and safety of single-port VATS lobectomy for early-stage lung cancer. Single-port VATS is just another variant of VATS surgery in the modern era. More time and effort is need to procure sufficient evidence to show that single-port VATS is more beneficial to patients compared with standard techniques, in terms of less trauma and less postoperative pain, without compromising ontological outcome.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kanematsu T, Hanibuchi M, Tomimoto H, et al. Epidemiological and clinical features of lung Cancer patients from 1999 to 2009 in Tokushima Prefecture of Japan. J Med Invest 2010;57:326-33. [PubMed]
- Lin Y, Cai L. Environmental and dietary factors and lung cancer risk among Chinese women: a case-control study in southeast China. Nutr Cancer 2012;64:508-14. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung Cancer. N Engl J Med 2013;368:1980-91. [PubMed]
- Murthy S. Video-assisted thoracoscopic surgery for the treatment of lung cancer. Cleve Clin J Med 2012;79 Electronic Suppl 1:eS23-5.
- Gonzalez-Rivas D. Evolving thoracic surgery: from open surgery to single port thoracoscopic surgery and future robotic. Chin J Cancer Res 2013;25:4-6. [PubMed]
- Rocco G. History and indications of uniportal pulmonary wedge resections. J Thorac Dis 2013;5:S212-3. [PubMed]
- Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung Cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Tseng YD, Cheng YJ, Hung MH, et al. Nonintubated needlescopic video-assisted thoracic surgery for management of peripheral lung nodules. Ann Thorac Surg 2012;93:1049-54. [PubMed]
- Rao PP, Rao PP, Bhagwat S. Single-incision laparoscopic surgery - current status and controversies. J Minim Access Surg 2011;7:6-16. [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [PubMed]
- Gonzalez-Rivas D, Fieira E, Mendez L, et al. Single-port video-assisted thoracoscopic anatomic segmentectomy and right upper lobectomy. Eur J Cardiothorac Surg 2012;42:e169-71. [PubMed]
- Gonzalez-Rivas D, Mendez L, Delgado M, et al. Uniportal video-assisted thoracoscopic anatomic segmentectomy. J Thorac Dis 2013;5:S226-33. [PubMed]
- Wang BY, Tu CC, Liu CY, et al. Single-incision thoracoscopic lobectomy and segmentectomy with radical lymph node dissection. Ann Thorac Surg 2013;96:977-82. [PubMed]
- Gonzalez-Rivas D. VATS lobectomy: surgical evolution from conventional VATS to uniportal approach. ScientificWorldJournal 2012;2012:780842.
- Gonzalez-Rivas D, Paradela M, Fieira E, et al. Single-incision video-assisted thoracoscopic lobectomy: initial results. J Thorac Cardiovasc Surg 2012;143:745-7. [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [PubMed]
- Gonzalez-Rivas D, de la Torre M, Fernandez R, et al. Video: single-incision video-assisted thoracoscopic right pneumonectomy. Surg Endosc 2012;26:2078-9. [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Uniportal video-assisted thoracoscopic pneumonectomy. J Thorac Dis 2013;5:S246-52. [PubMed]
- Rocco G, Martucci N, La Manna C, et al. Ten-year experience on 644 patients undergoing single-port (uniportal) video-assisted thoracoscopic surgery. Ann Thorac Surg 2013;96:434-8. [PubMed]
- Ng CS. Uniportal VATS in Asia. J Thorac Dis 2013;5:S221-5. [PubMed]
- Bertolaccini L, Rocco G, Viti A, et al. Geometrical characteristics of uniportal VATS. J Thorac Dis 2013;5:S214-6. [PubMed]
- Salati M, Brunelli A. Uniportal VATS for pneumothorax and interstitial lung disease. J Thorac Dis 2013;5:S217-20. [PubMed]
- Chen CH, Lee SY, Chang H, et al. Technical aspects of single-port thoracoscopic surgery for lobectomy. J Cardiothorac Surg 2012;7:50. [PubMed]
- Chen PR, Chen CK, Lin YS, et al. Single-incision thoracoscopic surgery for primary spontaneous pneumothorax. J Cardiothorac Surg 2011;6:58. [PubMed]
- Yang HC, Cho S, Jheon S. Single-incision thoracoscopic surgery for primary spontaneous pneumothorax using the SILS port compared with conventional three-port surgery. Surg Endosc 2013;27:139-45. [PubMed]
- Tander B, Ustun L, Ariturk E, et al. Balloon-assisted single-port thoracoscopic debridement in children with thoracic empyema. J Laparoendosc Adv Surg Tech A 2007;17:504-8. [PubMed]
- Wang BY, Liu CC, Shih CS. Short-term results of thoracoscopic lobectomy and segmentectomy for lung cancer in koo foundation sun yat-sen cancer center. J Thorac Dis 2010;2:64-70. [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [PubMed]
- Miyasaka Y, Oh S, Takahashi N, et al. Postoperative complications and respiratory function following segmentectomy of the lung - comparison of the methods of making an inter-segmental plane. Interact Cardiovasc Thorac Surg 2011;12:426-9. [PubMed]