Analysis of feasibility and safety of complete video-assisted thoracoscopic resection of anatomic pulmonary segments under non-intubated anesthesia
Introduction
Lung cancer is the most common cancer worldwide, accounting for about 15% of cancer cases around the world, and 28% of cancer deaths (1). Lung cancer is also associated with the highest morbidity and mortality among all malignant conditions in China (2). Surgical resection by thoracotomy or thoracoscopy is the preferred treatment for early-stage non-small cell lung cancer (3). Since the early 1990s, video-assisted thoracoscopic surgery (VATS) has been rapidly developed and widely applied in the world, involving almost all areas of general thoracic surgery. Compared with thoracotomy, VATS enables a smaller incision without removing or stretching the ribs open, sparing respiratory muscles from injures and thus minimizing the loss of lung function. Moreover, with a smaller incision, patients will suffer less pain postoperatively and expectorate more easily, reducing the incidence of postoperative pulmonary infection and complications as well (4). Thoracoscopic lobectomy is a representative application of thoracoscopic surgical techniques in thoracic surgery.
With the development and extensive application of imaging techniques such as high-resolution computed tomography (HRCT) and low-dose spiral computed tomography (CT), the detection rate of small lung nodule of unknown nature has been increasing. Lung resection is considered to be applicable for early lung cancer (T1N0M0), small metastases and localized benign lesions (such as bronchiectasis and tuberculosis) (5-8). Compared with lobectomy, segment resection better preserves lung functions while removing small nodules (9). With the intensified aging population, some patients are often complicated with cardiovascular diseases that make them unable to tolerate lobectomy, and therefore segmental resection has also been considered for the treatment of patients with primary lung cancer and poor cardiopulmonary function (3).
For now, general anesthesia with one-lung intubated ventilation is the standard anesthesia in thoracic surgery. Intubated anesthesia is, however, often associated with postoperative throat discomfort, including primarily irritating cough, and throat pain in some patients. On the other hand, non-intubated anesthesia can reduce general anesthesia-related complications, and many investigators have therefore begun to explore its application in general thoracic surgery. Dong et al. reported that thoracoscopic wedge resection under non-intubated anesthesia was feasible and safe (10). Chen et al. reported the safety and feasibility of thoracoscopic resection under non-intubated anesthesia (lobectomy, lung resection and wedge resection) in 285 patients (11). Hung et al. reported segmental resection under non-intubated anesthesia in 21 patients, finding that the technique preserved maximum normal lung tissue while reducing the loss of lung functions, and general anesthesia-related adverse reactions (12). This study summarizes 15 patients undergoing C-VATS resection of anatomic pulmonary segments under non-intubated anesthesia in our department.
Subjects and methods
Clinical data
Patients undergoing C-VATS resection of anatomic pulmonary segments from July 2011 to November 2013 were enrolled. All patients received pre-operative chest high-resolution thin-slice enhanced CT scans and pulmonary function tests. For those suspected of lung cancer, additional upper abdomen CT, head MRI, whole body bone scintigraphy or whole body PCT examination was needed to exclude distant metastases. Patients were eligible when they had an ASA grade of I-II, BMI <25 and no evident airway secretions or contraindications for epidural puncture in preoperative anesthesia assessment (11). All operations were performed by the same group of thoracic surgeons and anesthesiologist team. The primary outcome measures included the operative time, intraoperative blood loss, hospital stay, chest drainage, chest tube duration, and type of lung resection.
Indications for segmental resection
The indications for segmental resection included: (I) a lung mass close to the hilum in which wedge resection is not possible; (II) history of lung lobe resection, leading to the consideration of an additional primary lesion; (III) past history of other malignancies and lung solitary tumors, for which differentiation with primary lung cancer is not possible via intraoperative frozen sections; (IV) multiple pulmonary ground-glass shadows, for which atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS) or minimally invasive adenocarcinoma (MIA) may be suspected; (V) a complication with any cardiopulmonary disease that makes lobectomy intolerable; and (VI) peripheral early lung cancer ≤2 cm in diameter.
Surgical methods
Administration of anesthesia: with established intravenous rehydration, an epidural catheter is inserted in the thoracic T6-7 space. In the supine position, 2 mL of 2% lidocaine is injected through the epidural catheter. If signs of spinal anesthesia are not present in five minutes, fractionated injection of 12 mL 0.375% ropivacaine is administered. Before surgery, the anesthesia level should reach between T2 and T10. Propofol and remifentanil are infused for sedation and analgesia during surgery, with the BIS values maintained between 40 and 60. During surgery, masked and nasopharyngeal airway assisted ventilation is given with an inhaled oxygen concentration FiO2 of 0.33. Monitors are mounted on both sides along the patient’s head, which generally lies on the opposite side to the operating site, with the hilum and waist padded to further widen the intercostal space. The operator stands in front of the patient, the first assistant on the patient’s back side, and the second assistant handles the thoracoscope. The first port is generally made in the 7th or 8th intercostal space at the anterior axillary as the observation port. It should be noted that, in case that the diaphragm is too high or unclear on the X-ray images, this port should be positioned at a higher intercostal space to avoid injuring the abdominal organs. The second port is usually in the 7th intercostal space at the posterior axillary line and the third port close to the lesion, which form a triangle on the chest wall. All of them are treated with soft incision protectors to serve as the surgical operation channels. All video-assisted thoracic operations are performed using Stryker 1288 HD 3-Chip Camera/1288 with a three-chip HD camera system and specially designed endoscopic instruments in our department. After insertion of the thoracoscope from the first port, full chest exploration is conducted to determine whether there is evidence that the lesion is unresectable, such as pleural metastasis or other sign of metastases. Local vagus nerve block is achieved with 2 mL of 2% lidocaine under thoracoscopic guidance in the chest cavity, followed by spray of appropriate amount of the same concentration on the surface to reduce coughing that may induced by pulling of the lung tissue, ensuring a steady operation environment.
The thoracoscopic lung resection is done following the basic principle for lobectomy, in the order of arteries, bronchi, veins, and lung parenchyma in general. For resection of upper segments in the left upper lung, the veins are treated first because the superior branch of the superior pulmonary vein is anterior to, and blocks part of, its anterior branch, and thereby it should be first transected. The use of staplers and vascular clips is at the discretion of the operator depending on the vessel sizes during the surgery. According to the experience of the surgeons in our department, the use of hemolok and titanium clips should be avoided when clamping blood vessels. That is mainly because their application may affect the appropriate operation of other equipment such as stapler. (For example, a clip being caught in the stapler may prevent it from being successfully triggered.). Although in the event that vessels are well exposed, a stapler can be used to directly close or ligate and cut them off, there are still many factors that may affect those operations to such an extent that vessels are excessively pulled and injured when the stapler passes through them. In such cases, the tip of a linear stapler can be guided through the stapler guiding catheter to safely pass the posterior part of a vessel to successfully cut it off. The same method can be used to cut off bronchi, with satisfactory results. After the vessels and bronchi at the lesion segment are resected, the lung segment is in an atelectasis state. The anesthesiologist is instructed to maintain low volume low pressure ventilation to help determine the intersegmental plane. In addition, when the veins around the segment and in the surrounding segments to be preserved are well exposed, they can also be used to help identify the intersegmental plane. Mediastinal lymph node assessment is an essential component in thoracoscopic segmental resection for non-small cell lung cancer. Systemic lymph node dissection is performed following the segmental resection. Frozen sections of the segmental bronchus stumps and lymph nodes are sent for pathological tests. When positive intersegmental or interlobular metastases are present, switch to lobular resection is always preferred as long as the patient's physical conditions allow. If there is so little residual tissue following the resection that the high mobility makes lung torsion likely, Gossot et al. suggests connecting with the adjacent lobes via TA to reduce the postoperative complication (10). During surgery, if SpO2 drops to below 90%, mask assisted ventilation is needed to improve oxygenation. If blood gas analysis shows an arterial carbon dioxide partial pressure of ≥80 mmHg, the operation needs to be suspended followed by mask-assisted gas exchange. If the ventilation does not improve in this way, endotracheal intubation is required (9). Chest tube drainage is routinely used after the surgery. When there is no leakage and thoracic fluid volume is less than 200 mL per day, removal of the drainage can be considered.
Specific methods of segmental resection
(I) Resection of right upper posterior apical segments: the apical and posterior segments can be treated separately, but they are usually removed at the same time. The posterior ascending aorta anterior to the upper lobular bronchus is treated before the bronchi. The upper lobe is pulled forward to expose the posterior mediastinum. The pleura of the upper lobe bronchus close to the mediastinum are opened using coagulation hook, “peanut” gauze or a combination of both. A 45-mm endoscopic stapler is used to open the posterior part of the oblique fissure to help expose the ascending aorta, and the artery is transected. With combined use of the cautery hook, right-angle clamp and ultrasonic scalpel, the surrounding soft tissue is separated until the apical segmental bronchus is fully exposed. The apical artery is located posterior to it. A cutting stapler is used to close the bronchus while the posterior arteries are properly protected. After transection of the segmental bronchus, the apical artery is revealed. The upper lung lobe is pulled backwards to expose the apical vein anterior to the hilum, which is then closed and cut. When eventually cutting the lung parenchyma, the anesthetist is instructed to maintain low-pressure ventilation so that the boundary line between ventilated and non-ventilated areas can be followed as the cutting line.
(II) Resection of the upper segment in the right lower lung: with combined use of the coagulation hook and ultrasonic scalpel, the pleura around the hilum in the right lower lung are divided and the oblique fissure opened using a stapler. The pulmonary arteries are gradually exposed. After the upper segmental artery is divided and cut, the posterior bronchus is revealed, separated, stapled and cut. The inferior pulmonary ligament is transected through to the inferior pulmonary vein. Gauze is used to expose the superior segmental vein upwards from the inferior pulmonary vein, and the former is then cut with a vascular clamp or stapler.
(III) Resection of the basal segment in the right lower lung: the anterior part of the oblique fissure is opened to expose the basal segment artery, which is transected and closed. The segmental bronchus is separated from the deep structure of the artery. The anesthesiologist is instructed to help identify if the basal segment bronchus is closed off by ventilation. The inferior pulmonary ligament is transected through to the inferior pulmonary vein. With the inferior lobe is pulled up, the surrounding tissue of the inferior pulmonary vein is divided using the cautery hook and peanut gauze. The basal segment vein is exposed and transected.
(IV) Lingular segment of the left upper lung: the lingular artery is separated and transected to reveal the upper lobular bronchus and lingular segmental bronchus. The latter is clamped, and low ventilation is used to identify its closure before transaction. The superior pulmonary vein is separated until its lowermost branch is exposed. If the lingular segmental vein can be located, it is transected before the intersegmental pulmonary tissue is handled. Otherwise, the lingular segmental vein can be treated until the lingular segmental tissue is fully separated.
Results
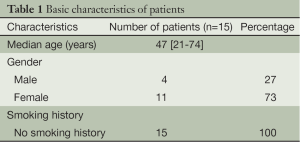
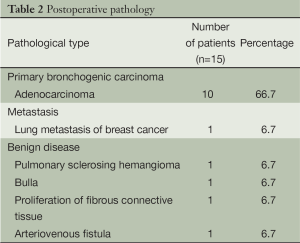
The procedures were successfully completed in 15 patients, including four men and eleven women. The average age was 47 [21-74] years. The patient characteristics are listed in Table 1. Pathological examination showed ten patients with adenocarcinoma, one with pulmonary metastases, and four with benign lung lesions (Table 2).
Full table
Full table
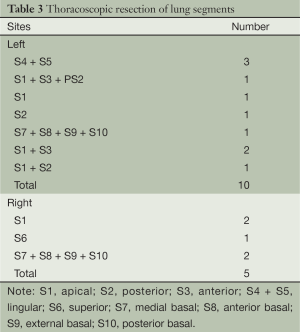
Segmental resections were successful in all patients without switching to thoracotomy or lobectomy. The resected sites included: right upper apical segment, two; right lower dorsal segment, one; right lower basal segment, two; left upper lingular segment, three; left upper apical segment, one; left upper anterior apical segment, two; left upper posterior segment, one; left lower basal segment, one; left upper posterior and apical segments, one; and left upper anterior and apical segments plus wedge resection of the posterior segment, one. Resected lung segments are shown in Table 3.
Full table
One case had intraoperative bleeding of 1,450 mL, which was controlled with thoracoscopic operation and no blood transfusion was required. There were no perioperative deaths. Two patients of postoperative bleeding were controlled with hemostatic medicine without the need for blood transfusions, and no other serious complications occurred. All patients were cured and discharged. The overall mean operative length was 166 minutes (range 65-285 minutes), mean blood loss 75 mL (range 5-1,450 mL), mean postoperative chest drainage 294 mL (range 0-1,165 mL), mean chest drainage time 2 days (range 0-5 days), and mean postoperative hospital stay 5 days (range 3-8 days) (Table 4).
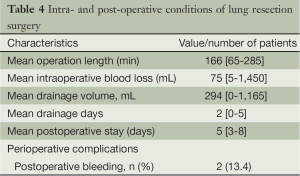
Full table
Of the ten patients with primary lung cancer, nine received mediastinal lymph node dissection or systemic lymph node sampling, and the pathological staging showed stage IA for them; one patient who did not receive the above procedure had micro invasive aden
ocarcinoma in the left lung. After 4-19 months of follow-up for the patients, no tumor recurrence and metastasis was found.
Discussion
Whether segmental resection can achieve comparable effects to lobectomy for the treatment of early stage lung cancer is still controversial. Previous studies have shown that for early lung cancer, particularly when the tumor diameter is ≤2 cm, segmental resection can yield comparable long-term survival as with lobectomy (13,14). However, evidence in this regard comes mainly from retrospective case comparisons and meta-analyses, and the role of segmental resection in NSCLC needs to be further confirmed by large international multi-center randomized controlled clinical studies (CALGB 140503 in the United States and JCOG0802/WJOG4607L in Japan).
Complete thoracoscopic segmental resectionis a complex and technically demanding procedure, requiring the surgeon to be extremely familiar with the anatomic structures of every segmental vessel and bronchus. One of the major technical difficulties is confirmation of the plane between segments. Most investigators traditionally suggest low-pressure ventilation after occlusion or transection of segmental bronchi, so that the plane can be determined by differentiating between the collapsed and expanded interface. The purpose of the ventilation is to avoid the influence on endoscopic vision and operation by excessive expansion of lung tissue. According to our experience, a long-handled tong may be used to clamp the plane after low-pressure ventilation, as it provides two main advantages: (I) in view of the traffic between the lung segments, adjacent lung segments can be expanded with ventilation, blurring the lung segment boundary; (II) a stapler only provides a limited opening angle that is likely to injury the lung parenchyma when coming across the thicker portion of it, leading to the need of manual stitches and bleeding control after the resection, which will increase the length of operation. The use of this recommended instrument can provide local compression, making it easier for a stapler to pass the lung segment boundary. Some investigators on the other hand suggest the use of selective lung ventilation in patients with COPD, in which the target segment is expanded through bronchoscopy and separated from other collapsed lung segments, reducing the impact of endoscopic vision by lung expansion (15). Segmental veins can also be helpful in identifying the intersegmental plane, and separation along pulmonary veins and loose connective tissue in the lung segments usually does not damage large bronchi and pulmonary arterial branches. Some lesions are located between segments, and when reliable surgical margins are not secured, resection of the adjacent segments can be considered.
Compared with traditional surgery under general anesthesia, epidural analgesia reduces intubation-related complications and facilitates early mobility of patients (10,11,16). It also reduces the dose of intraoperative anesthesia drugs, which will help restore the breathing and digestive functions. Four to six hours after non-intubation segmental resection, the patients could start eating, drinking, and get out of bed. Chest X-ray scans could be performed on the same the day after surgery. If imaging tests suggest good lung recruitment and no air leaks, and 24-h chest drainage is less than 200 mL, the drainage can be removed. With non-intubated anesthesia, coughing induced by postoperative throat discomfort is significantly reduced. Coughing may worsen wound pain, which in turn suppresses the cough reflex, making pulmonary secretions difficult to discharge after surgery, and indirectly leading to alveolar hypoventilation due to rapid and shallow breathing; some patients may even experience atelectasis or lung infection after surgery. Therefore, non-intubation endoscopic resection of lung segments may reduce the incidence of pulmonary complications, maximize protection of lung function and reduce postoperative pain, shorten chest tube duration, shorten the length of hospital stay, and allow faster recovery to preoperative mobility.
Non-intubated anesthesia combined with C-VATS lung resection surgery should be one of the most minimally invasive lung cancer surgery at present. With non-intubation anesthesia, the biggest challenge for surgeons is the remarkable mediastinal motion, which requires full cooperation among the surgeon, anesthetists and assistants. Mediastinal movement occurs when the ipsilateral intrathoracic pressure was significantly higher than that of the contralateral side in open pneumothorax, resulting in mediastinal shift to the contralateral area that further limits expansion of the contralateral lung. During inhalation and exhalation, the unbalanced pleural pressure on both sides experiences cyclical changes so that the contralateral mediastinum moves toward the contralateral side during inhalation and the opposite side during exhalation. In non-intubation segmental resection, the patient’s spontaneous breathing has to be retained in order to achieve atelectasis of the operative side and good ventilation of the contralateral lung, so that both the oxygen supply and a favorable operating field can be secured. With collapsed ipsilateral lung after thoracotomy, some patients will have obvious mediastinal swing, which will affect the surgeon’s surgical operation, particularly when dealing with blood vessels in which excessive traction may lead to bleeding. To mitigate the impact of the mediastinal swing during surgery, anesthesiologists can increase the amount of opioids based on the operation, reduce the breathing frequency or the respiratory tidal volume, thereby reducing the amplitude of the swing. At the same time, appropriate ventilation can be given based on the results of blood gas analysis to avoid serious hypercapnia, so as to maintain the body’s acid-base balance.
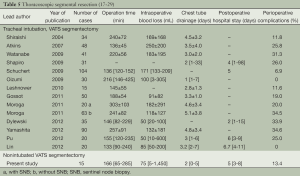
Based on the fifteen patients undergoing non-intubated anesthesia combined with C-VATS lung resection in our department, the technique is feasible and safe with the help of skilled anesthetists with experience in thoracoscopic lobectomy and non-intubated anesthesia. So far, there has been no shift to thoracotomy and lobectomy. Although there was one case of bleeding, it was well controlled endoscopically without the need of blood transfusion. As for the two cases of postoperative bleeding, no blood transfusions were needed and no other complications were observed. The incidence of perioperative complication was 13.4%. The mean operative time was 166 minutes, mean intraoperative blood loss 75 mL, mean postoperative chest drainage two days, and mean postoperative hospital stay five days. The operative time and the number of days in hospital are comparable to those reported with VATS under general anesthesia, while intraoperative blood loss, chest drainage time and perioperative complications were better than the latter (Table 5).
In summary, complete video-assisted thoracoscopic surgery (C-VATS) under non-intubated anesthesia for the resection of anatomic pulmonary segments in the treatment of early lung cancer (T1N0M0), benign lung diseases and lung metastases is safe and feasible, and can reduce postoperative pain, improve the appearance with small incisions, shorten chest drainage duration and postoperative hospital stay, provide maximum protection of lung functions, and reduce complications after general anesthesia. However, it requires that the surgeon has extensive experience in thoracoscopic lung resection in good cooperation with anesthesia doctors. Due to the short follow-up period, the long-term efficacy needs to be further confirmed. The long-term effect of non-intubated thoracoscopic anatomic segmental resection needs to be further studied and identified in a larger-scale study.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- American cancer Society, Lung Cancer Statistics, 2010.
- Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx
- Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw 2013;11:645-53. [PubMed]
- He J, Xu X. Thoracoscopic anatomic pulmonary resection. J Thorac Dis 2012;4:520-47. [PubMed]
- Shapiro M, Weiser TS, Wisnivesky JP, et al. Thoracoscopic segmentectomy compares favorably with thoracoscopic lobectomy for patients with small stage I lung cancer. J Thorac Cardiovasc Surg 2009;137:1388-93. [PubMed]
- Zhong C, Fang W, Mao T, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg 2012;94:362-7. [PubMed]
- Donahue JM, Morse CR, Wigle DA, et al. Oncologic efficacy of anatomic segmentectomy in stage IA lung cancer patients with T1a tumors. Ann Thorac Surg 2012;93:381-7; discussion 387-8. [PubMed]
- Xiong X, Shao W, Yin W, et al. Video-assisted thoracoscopic surgery for stage I non-small cell lung cancer: long-term survival and prognostic factors. Tumour Biol 2013; [PubMed]
- Yoshimoto K, Nomori H, Mori T. J Thorac Cardiovasc Surg 2009;137:1200-5. [PubMed]
- Dong Q, Liang L, Li Y, et al. Anesthesia with nontracheal intubation in thoracic surgery. J Thorac Dis 2012;4:126-30. [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Hung MH, Hsu HH, Chen KC, et al. Nonintubated thoracoscopic anatomical segmentectomy for lung tumors. Ann Thorac Surg 2013;96:1209-15. [PubMed]
- Read RC, Yoder G, Schaeffer RC. Survival after conservative resection for T1 N0 M0 non-small cell lung cancer. Ann Thorac Surg 1990;49:391-8; discussion 399-400. [PubMed]
- Koike T, Togashi K, Shirato T. Ann Thorac Surg 2009;88:1106-11. [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique:Selective segmental inflation via bronchofiberoptic jet followed by cautery cutting . J Thorac Cardiovasc Surg 2007;133:753-8. [PubMed]
- Shao W, Wang W, Yin W, et al. Nonintubated thoracoscopic lobectomy plus lymph node dissection following segmentectomy for central type pulmonary masses. Chin J Cancer Res 2013;25:124-7. [PubMed]
- Shiraishi T, Shirakusa T, Iwasaki A, et al. Video-assisted thoracoscopic (VATS) segmentectomy for small peripheral lung cancer tumors. Surg Endosc 2004;18:1657-62. [PubMed]
- Atkins BZ, Harpole DH Jr, Mangum JH, et al. Pulmonary segmentectomy by thoracotomy or thoracoscopy: reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg 2007;84:1107-12; discussion 1112-3. [PubMed]
- Watanabe A, Ohori S, Nakashima S, et al. Feasibility of video-assisted thoracoscopic surgery segmentectomy for selected peripheral lung carcinomas. Eur J Cardiothorac Surg 2009;35:775-80; discussion 780. [PubMed]
- Shapiro M, Weiser TS, Wisnivesky JP, et al. Thoracoscopic segmentectomy compares favorably with thoracoscopic lobectomy for small stage I lung cancers. J Thorac Cardiovasc Surg 2009;137:1388-93. [PubMed]
- Schuchert MJ, Pettiford BL, Pennathur A, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg 2009;138:1318-25.e1.
- Oizumi H, Kanauchi N, Kato H, et al. Total thoracoscopic pulmonary segmentectomy. Eur J Cardiothorac Surg 2009;36:374-7; discussion 377. [PubMed]
- Leshnower BG, Miller DL, Fernandez FG, et al. Video-assisted thoracoscopic surgery segmentectomy: a safe and effective procedure. Ann Thorac Surg 2010;89:1571-6. [PubMed]
- Gossot D, Ramosa R, Brian E, et al. A totally thoracoscopic approach for pulmonary anatomic segmentectomies. Interact Cardiovasc Thorac Surg 2011;12:529-32. [PubMed]
- Moroga T, Yamashita S, Tokuishi K, et al. Thoracoscopic segmentectomy with intraoperative evaluation of sentinel nodes for stage I non–small cell lung cancer. Ann Thorac Cardiovasc Surg 2012;18:89-94. [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [PubMed]
- Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg 2012;42:83-8. [PubMed]
- Pu Q, Mei JD, Liao H, et al. Complete video-assisted thoracoscopic anatomic segmentectomy for pulmonary diseases: the early experiences. Zhonghua Wai Ke Za Zhi 2012;50:823-6. [PubMed]
- Lin ZW, Jiang W, Wang Q, et al. Total thoracosenpic anatomic pulmonary segmentectomy for 20 patients. Chin J Clin Thorac Cardiovasc Surg 2012;19:270-3.