Sleeve lobectomy for lung adenocarcinoma treated with neoadjuvant afatinib
Introduction
Sleeve lobectomy was initially developed for lung cancer patients with insufficient pulmonary reserve, and is now widely accepted as a reliable and safe procedure to allow complete resection (1). Sleeve resection has also reported to be safe and have some advantages even after neoadjuvant therapy (2). However, there is limited data of sleeve lobectomy with neoadjuvant epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI). Here we describe the case of sleeve lobectomy for lung adenocarcinoma treated with neoadjuvant afatinib. The clinical course, treatment and pathological findings of the patient as well as the potential of EGFR-TKI to avoid pneumonectomy are discussed.
Case presentation
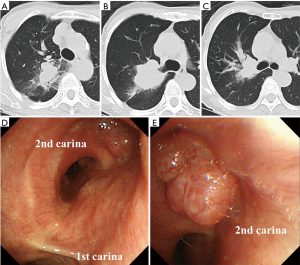
A 70-year-old previously healthy woman (32 kg, 138 cm) who was a current smoker (50 pack-year) was referred to our department for lung resection of an adenocarcinoma of the right upper lobe invading the second carina. The adenocarcinoma harbored an EGFR mutation (exon 19 deletion). Spirometry showed a slightly reduced forced expiratory volume in 1 second (FEV1) value of 1.46 L (84.2% of the predicted value). Computed tomography (CT) (Figure 1A,B,C) and bronchoscopy (Figure 1D,E) demonstrated a heterogeneous mass, 52 mm in size, at the orifice of the right upper lobe. 18F-fluorodeoxyglucose (FDG)-positron emission tomography showed uniform high accumulation of FDG (standardized uptake value: 9.8) in the mass, while no significant accumulation was observed in the lymph nodes (cT3N0M0). Considering the location of the tumor, reduced FEV1 value, and the presence of EGFR mutation, the patient was planned to be prescribed afatinib (30 mg daily) for 3 weeks as neoadjuvant therapy and underwent sleeve lobectomy to avoid pneumonectomy as much as possible.
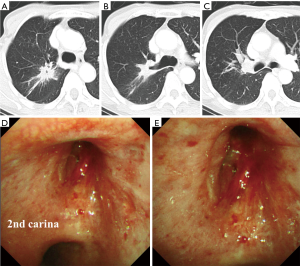
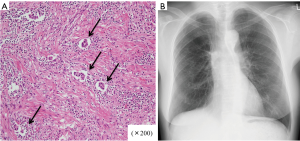
Although the patient presented with grade 3 diarrhea and dose reduction of afatinib to 20 mg daily was needed, CT (Figure 2A,B,C) and bronchoscopy (Figure 2D,E) showed a partial response of the tumor on Day 20. Oral administration of afatinib was discontinued on Day 22. Right upper sleeve lobectomy combined with partial resection of lower lobe with systematic mediastinal lymph node dissection was performed through right lateral thoracotomy on Day 24. The bronchial margin was confirmed to be free of tumor cells by intraoperative pathological evaluation of frozen sections. The patient’s postoperative course was uneventful and she was discharged on postoperative Day 6. Pathologic examination revealed residual malignant cells in the center of the mass, which was less than one-third in diameter of the total size including the surrounding fibrous scar (Figure 3A) with no lymph node involvement, and the resection margins of the bronchus were negative for malignancy. The patient has been free of recurrence for 26 months after surgery (Figure 3B).
Discussion
Several surgical procedures have been reported to perform complete resection while minimizing the loss of pulmonary reserve (1-4). In addition, a previous report showed the safety and advantages of sleeve resection after neoadjuvant therapy to date (2). In the present case, neoadjuvant afatinib was administrated for 3 weeks because of the presence of EGFR mutation and the location of the tumor, to reduce the risk of pneumonectomy as much as possible.
Previous studies have shown that neoadjuvant treatment with first-generation EGFR-TKIs in early non-small cell lung cancer (NSCLC) has low toxicity in limited populations, thus it has potential to use in clinical settings with acceptable adverse effects (5,6). The second-generation EGFR-TKI afatinib is an oral, selective and irreversible ErbB family blocker (EGFR, HER2 and HER4), which has been postulated to be associated with improved inhibition of EGFR-dependent tumor growth compared with first-generation EGFR-TKIs for advanced NSCLC harboring activating EGFR mutations (7). Although the overall survival has been reported to be similar between first-generation TKIs and second-generation TKI, the progression free survival and objective response rate were significantly improved with afatinib in patients with EGFR deletion 19, with a manageable safety profile (8). Ongoing clinical studies will help to define the activity of afatinib in other setting, including adjuvant and neoadjuvant treatment (NCT01553942) of mutant NSCLC patients, however, to date, the role of afatinib in neoadjuvant setting is still not demonstrated.
Comparing afatinib and other anticancer agents such as platinum-based chemotherapy in terms of bronchial anastomosis, previous study demonstrated that chemoradiotherapy had a negative effect on bronchial blood supply more than chemotherapy alone, leading to an increased risk of anastomotic complications (9). Considering these reported data and biological mechanism of afatinib, there might be little risk to use afatinib instead of chemo- or chemoradiotherapy.
Although previous phase I studies reported that the recommended dose of afatinib was 50 mg daily in patients with advanced NSCLC, the initial dose of the present case was 30 mg daily because of lower body weight (32 kg) (10). Furthermore, diarrhea, a drug-related adverse event, was observed, and dose reduction of afatinib was needed; however, a favorable tumor response was confirmed in the preoperative period, and complete resection by sleeve lobectomy was successfully performed. Considering afatinib has been reported to be well tolerated and effectively managed using predefined dose modification schemes (7), it might be considered as a treatment option in neoadjuvant settings for patients with EGFR mutations. The result of the above-mentioned ongoing trial is awaited and further evidence is needed to assess the effectiveness of afatinib as neoadjuvant therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Nagayasu T, Yamasaki N, Tsuchiya T, et al. The evolution of bronchoplasty and broncho-angioplasty as treatments for lung cancer: evaluation of 30 years of data from a single institution. Eur J Cardiothorac Surg 2016;49:300-6. [Crossref] [PubMed]
- Storelli E, Tutic M, Kestenholz P, et al. Sleeve resections with unprotected bronchial anastomoses are safe even after neoadjuvant therapy. Eur J Cardiothorac Surg 2012;42:77-81. [Crossref] [PubMed]
- Okada M, Tsubota N, Yoshimura M, et al. Extended sleeve lobectomy for lung cancer: The avoidance of pneumonectomy. J Thorac Cardiovasc Surg 1999;118:710-3; discussion 713-4. [Crossref]
- Oto T, Kiura K, Toyooka S, et al. Basal segmental auto-transplantation after pneumonectomy for advanced central lung cancer. Eur J Cardiothorac Surg 2012;42:579-81. [Crossref] [PubMed]
- Lara-Guerra H, Waddell TK, Salvarrey MA, et al. Phase II study of preoperative gefitinib in clinical stage I non-small-cell lung cancer. J Clin Oncol 2009;27:6229-36. [Crossref] [PubMed]
- Schaake EE, Kappers I, Codrington H, et al. Tumor response and toxicity of neoadjuvant erlotinib in patients with early-stage non-small-cell lung cancer. J Clin Oncol 2012;30:2731-8. [Crossref] [PubMed]
- Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. [Crossref] [PubMed]
- Paz-Ares L, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270-7. [Crossref] [PubMed]
- Hampel M, Dally I, Walles T, et al. Impact of neo-adjuvant radiochemotherapy on bronchial tissue viability. Eur J Cardiothorac Surg 2010;37:461-6. [PubMed]
- Eskens FA, Mom CH, Planting AS, et al. A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br J Cancer 2008;98:80-5. [Crossref] [PubMed]


