Right anterior mini-thoracotomy vs. conventional sternotomy for aortic valve replacement: a propensity-matched comparison
Introduction
Minimally invasive aortic valve replacement (MIAVR) through a right mini-thoracotomy is an interesting approach introduced in 1993 (1). By 1996, various techniques have been developed, with a wide variety of incisions including partial lower and transverse sternotomies as well as a parasternal approach being proposed (2). Nowadays, right anterior thoracotomy and upper hemi-sternotomy are increasingly used strategies to perform minimally invasive AVR. Literature data associate the minimally invasive surgery to less bleeding, shorter duration of mechanical ventilation, and reduced intensive care unit and hospital stay despite longer procedure times, together with an expected improved cosmetic result and a reduction in wound infections (3-8). On the other hand, major limitations included a very tiny operating field, resulting in longer operating times compared to the standard approach, and the need for peripheral cannulation (9-11).
In order to better evaluate the potential benefits of MIAVR a standardization of the procedure itself is required. For this reason, data from a single group using a standardized technique would be helpful. Consequently, we collected data about our experience on MIAVR in order to compare it with the conventional “gold standard” full sternotomy performed by the same surgical team.
Methods
Demographic, intraoperative and outcome data of all patients were collected in the clinical database and accurately verified for completeness and accuracy against the patients’ clinical charts.
The study protocol was approved by the local Ethic committee (Comitato Etico Area Vasta Romagna CEIIAV with number 1189) and each patient signed an informed consent for the treatment of personal data.
Patients selection
Between January 2010 and May 2016, 1,180 patients with symptomatic aortic stenosis underwent AVR in two cardio surgical Italian centres managed by one single team of Cardiac Surgeons (Maria Cecilia Hospital, Cotignola, main Center; Villa Torri Hospital, Bologna, spoke Center). The decision to use or not MIAVR was left to surgeon’s preference; as a guiding strategy, the only exclusion criterion for MIAVR was considered a history of previous left pneumonectomy. Consequently, the latter group was not included in the registry.
Demographic, intraoperative and short-term outcome data of all patients were collected and included in the registry. MIAVR procedures were performed only by trained operators who had a surgical experience with mini-invasive approaches comparable to the one with conventional sternotomy. No residents or fellows did perform either MIAVR or conventional sternotomy procedures.
Surgical technique
All patients undergoing MIAVR received a totally intravenous anesthesia, as well as those treated with full sternotomy, and an intubation with a double lumen endotracheal tube was needed. Transesophageal echocardiography was used in all patients for monitoring heart and valve function during the operation.
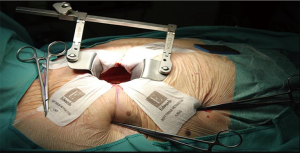
A pillow positioned under the right shoulder was used. In MIAVR patients, a 4- to 6-cm skin incision at the 3rd right intercostal space (midclavicular line) was performed. The soft tissue retractor (CV MICS Sorin Group, the Edwards ThruPortTM Systems or the Covidien SurgiSleeve) was used to help spreading the chest wall. The pericardium was opened 4–5 cm cranially of the phrenic nerve, over the right ventricle. Three deep stay sutures were pulled towards the operator to obtain best surgical exposure. Sutures were tightened using the Endo close trocar site closure device (Covidien, Mansfield, MA, USA) outside the chest wall (Figure 1).
At the beginning of our experience a peripheral cannulation was adopted to increase familiarity with the “new” surgical approach” and to get a better operating field overview (42 patients). Nowadays, we prefer and suggest a total central cannulation avoiding the peripheral one (12). In case of needing to switch to a conventional full sternotomy, patients were first weaned form cardio-pulmonary by-pass; then, sternotomy was performed and cardio-pulmonary by-pass start over again.
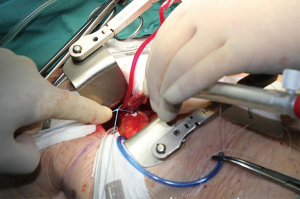
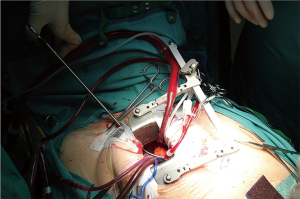
Purse string sutures were placed before systemic heparinization treatment to avoid hematoma of ascending aorta wall and to reduce the potential blood loss. A straight arterial cannula (EOPA arterial cannula, Medtronic, Inc. Minneapolis, Minn or a Straightshot Edwards Lifesciences in a minority of cases) was positioned just below the origin of aortic arch, so that the Chitwood clamp, positioned percutaneously, did not hinder the operating field, while the aortic annulus was brought closer to the operator (Figure 2). The venous purse string was placed around the left appendage and reinforced by pledgets. A three-stage MC2X venous cannula (Medtronic, Inc. Minneapolis, Minn) was placed using the insider of left ventricle vent to facilitate the insertion. The stitch used to fix the cannula was then pulled outside the chest using the Endo close, trocar site closure device, thus improving exposure moving the appendage and the right atrium away from the aortic root (Figure 3). The antegrade flow cannula was inserted in the ascending aorta as routinely; the ventricle was vented through the right upper pulmonary vein (DLP 20 Fr, ref. 12002, Medtronic). The aorta was cross-clamped through a 1-cm skin incision made at the origin of the innominate vein using a Chitwood clamp. Hypothermic 6 °C blood cardioplegia (St Thomas with procaine 0.68 mL/kg) was administered in an antegrade fashion into the aortic root to stop the heart. A normothermic cardiopulmonary bypass was chosen for all patients. A transverse incision of the ascending aorta was then performed and the aortic valve was removed. Whenever it was possible, the native aortic valve was excised in a single step, reducing time for decalcification. The prosthesis was then sized. During the rinsing of the valve, the first stitch (4-0 polipropilene) to close the transverse aortotomy was positioned. Prosthetic valve was implanted using three running 2-0 polypropylene sutures starting from the annulus below the right coronary ostium and moving then to the annulus below the left and the non-coronary sinuses. This technique was chosen to reduce cross-clamp times.
Ascending aorta incision was sutured and ventricular pacing wires placed on the right ventricle. Aorta was then declamped and the patient weaned from cardiopulmonary bypass. Intraoperative transesophageal echocardiography was used to assess the correct prosthesis function and competence. Cannulas were removed and protamine was administered at 1:1 ratio to heparin.
In case of full sternotomy approach the technique and the devices adopted for extracorporeal circulation and for prosthesis implantation were absolutely the same. The only differences consisted in the absence of double lumen intubation and in patient position.
Propensity matching
Propensity matching was performed in order to create two groups with no difference with respect to pre-surgery characteristics, i.e. confounding factors. Propensity score was estimated with a logistic regression model where the treatment group was the outcome and the explanatory variables where those associated to both the treatment and the in-hospital mortality: ejection fraction, presence of diabetes, creatinine clearance and logistic euro score. Matched sample was obtained according to the nearest neighbour procedure: each patient in MIAVR group (treated) was matched with one patient in full sternotomy group (control) with similar propensity score, where similar means a maximum difference in propensity score of 10% of its standard deviation. At each step the procedure match the control patient that was not yet matched but was closest to the treated patient; if none of the controls satisfied the similarity condition, the treated patient was discarded from the matched sample.
A maximum difference of 10% of the propensity score standard deviation resulted in a maximum propensity score difference of 0.014 (2%), that is the propensity score of the matched control was in the range ±0.014 of propensity score of the treated patient. This caliper drove to a matched sample of 363 patients per each treatment group. Balance check was done measuring the % improvement in balance measure after the matching. Balance measure was based on mean difference between treated and control group for each explanatory variable. The resulting improvement was ≥90% for each covariate.
Statistical analysis
Continuous data were tested for normal distribution with the Kolmogorov–Smirnov test. Normally distributed values were presented as mean ± SD and were compared by t-test; otherwise median value (interquartile range) and Mann-Whitney U test were used. Categorical variables were summarized in terms of number and percentages and were compared by two-sided Fisher’s exact tests. A multivariable logistic regression model was fitted to identify independent predictors of in-hospital mortality and included main clinical variables (age, sex, BMI, diabetes, COPD, renal function and EuroSCORE) as well as procedural times (CPB time and clamping time) and the surgical approach (MIAVR vs. conventional sternotomy).
All tests were two sided, and the statistical significance was defined as P value <0.05. All analyses were performed using the statistical software SPSS version 20 (SPSS, Inc., Chicago, Illinois, USA) and R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
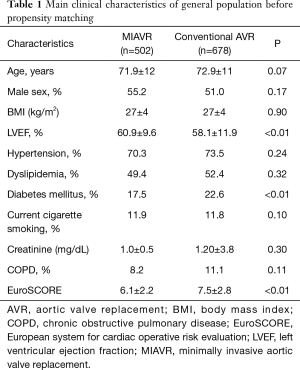
Overall, 678 (57%) patients were treated with a standard full sternotomy approach, while 502 (43%) patients underwent MIAVR using a right minithoracotomy. Baseline characteristics of patients included in the registry are described in Table 1. Of note, patients addressed to MIAVR were at lower global risk with a EuroSCORE significantly lower compared to conventional AVR patients (6.1±2.2 vs. 7.5±2.8; P<0.01) and a lower proportion of diabetic patients (17.5% vs. 22.6%, P<0.01).
Full table
After propensity matching of all patients undergoing AVR, we identified 726 patients (363 patients per each group) well balanced for baseline characteristics (Table 2).
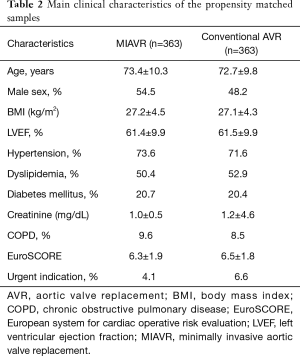
Full table
Overall procedure duration was significantly higher in MIAVR patients compared to conventional sternotomy patients (195.1±56.8 vs. 167.1±47.2 min, respectively; P<0.001). However, cardiopulmonary bypass time was significantly lower in MIAVR group (61.0±21.0 vs. 65.9±24.7 in conventional sternotomy group; P<0.01). Similarly, aortic cross clamp times were significantly lower in MIAVR group compared to sternotomy group (48.3±16.7 vs. 53.2±19.6 min, respectively; P<0.01).
In MIAVR group intraoperative conversion to full sternotomy was required in two patients due to paravalvular leakage which was not safely fixable through the minithoracotomy approach.
In MIAVR patients all models and sizes of available prostheses were implanted. A biological prosthesis was implanted in all patients of the propensity-selected MIAVR group. Implanted prostheses models and sizes with regard to MIAVR group are summarized in Table 3.
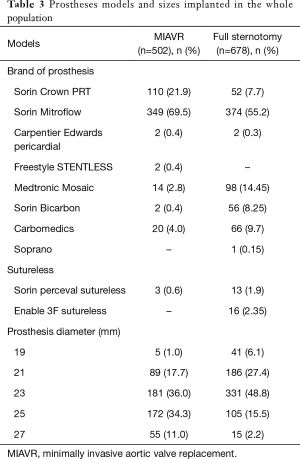
Full table
Main clinical and operative outcomes in the two groups are reported in Table 4. Of note, no significant differences in major outcomes were observed between the two groups. In-hospital mortality was not significantly different between the groups (1.7% in MIAVR patients vs. 2.2% in conventional sternotomy patients; P=0.79). Besides reported outcomes, one patient in conventional AVR group had to be reoperated due to early endocarditis.
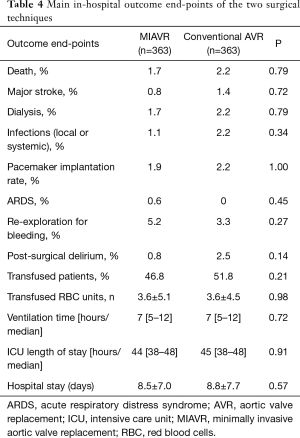
Full table
Post-operative duration of ICU stay was not different between the two groups (median 44, IQ range 38–48 hours in MIAVR patients vs. median 45, IQ range 38–48 hours in conventional sternotomy patients; P=0.91),
Nearly half of the patients were discharged home. The remaining patients were referred to our rehabilitation unit or transferred to another hospital facility to complete the recovery. No difference was wound between the two surgical approaches with regard to the length of hospital stay (Table 4).
Predictors of in-hospital mortality
Univariable and multivariable predictors of mortality are summarized in Table 5. Although both longer cardiopulmonary bypass times and aortic cross clamp times were associated with mortality, only EuroSCORE (OR 1.52, 95% CI: 1.12–2.06; P<0.01) was found to be an independent predictor of intra-hospital mortality in the whole sample.
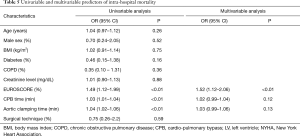
Full table
Discussion
At the beginning of 2010 we introduced the right minithoracotomy to treat isolated aortic valve replacement, as an alternative minimally invasive approach with the aim to reduce operation discomfort. Currently available about clinical outcome and operative results of this approach mainly come from multi-center experiences putting together patients with different characteristics and not completely reproducible techniques (upper ministernotomy in most, right anterior minithoracotomy in a few) (13,14). In order to obtain reliable and reproducible results, a standardized and reproducible technique, possibly coming from single high-volume centers sharing the same surgical approach, is mandatory.
This study describes a large experience coming from two centers run by the same surgical team, with a standardized approach to perform MIAVR.
Our main findings are the following:
- MIAVR through right anterior mini-thoracotomy is safe and feasible with a standardized approach, with all prostheses size and type implanted;
- Although operative times are higher, cross clamping and extracorporeal times were lower than those in conventional sternotomy patients;
- Propensity-matched comparison showed that in-hospital outcomes were comparable to those obtained with full sternotomy.
Potential advantages of MIAVR arise from the concept that patient morbidity and potential mortality could be reduced without compromising the excellent results of the conventional procedure with improved cosmetic results, safer access in the case of reoperation, less post-operative bleeding, fewer blood transfusions, lower intensive care unit and in-hospital stays (15). The major criticism reported regard the very tiny operating field, resulting in longer operating times compared to the standard approach. Actually, our results showed a significantly longer overall procedure duration (skin-to-skin) in MIAVR patients compared to conventional full sternotomy patients due to a meticulous preparation of the operative field, as described. This paradoxically resulted in lower operative times (both cardio-pulmonary bypass and cross clamp times) in MIAVR group. This might be relevant even for clinical outcome, since we found a significant (but not independent) association of both operative times with in-hospital mortality, which has been previously demonstrated in patients receiving sutureless prostheses (16). This confirms the need for reducing cross-clamping times by using standardized procedures and an adequate learning-curve (17). Actually, our experience in a large cohort of patients and the meticulous preparation of the operative field made MIAVR patients’ risk not different from the conventional sternotomy group.
The second crucial point often cited is the need of peripheral cannulation that it is not free from complications (9-11). After a correct learning curve that can be applied using a femoral cannulation, a total central one can be easily adopted with the standard straight aortic cannula. Pulling on the venous cannula it is possible to obtain a minimal amount of space; in addition, the costal avulsion and right mammary artery interruption is avoidable by moving the retractor laterally, where the intercostal space is wider. Of note, no preoperative CT scan was needed in MIAVR patients. In contrast with Glauber et al. (18), we don’t think that a preoperative CT scan is necessary to analyze the thoracic “architecture” and the heart anatomy, since the ascending aorta can be easily assessed to evaluate the presence of calcified plaques in order to choose the best location for cannulation and clamp, as surgeons usually do in full sternotomy, without an increase in the embolic risk as demonstrated by the low incidence of neurologic events in our experience (0.8%).
While other reports described shorter ventilation times with MIAVR use (16), our experience showed no differences between the two groups. As a fact, this can be explained by the choice of not treating differently the two groups in the post-operative period: our Hospital decided to follow a standard pathway which did not take into account the surgical technique used. A tailored approach would be encouraged for the future, in order to better evaluate post-operative recovery in the two groups according to the different surgical strategy.
Similarly, since smaller incisions should theoretically reduce post-operative bleeding and transfusion requirements, other groups reported a lower need for transfusions in MIAVR patients (10). However, other reports from isolated studies showed no differences in transfusion requirements (19,20). In agreement with the latter observations, in our series there was no difference in the number of transfused units between MIAVR and conventional sternotomy patients, while a non-significant trend of a reduced number of patients requiring transfusions in MIAVR group was observed.
In conclusion, MIAVR through right anterior minithoracotomy is a safe and effective surgical strategy that avoids sternotomy and rib fracture, guaranteeing cosmetic and functional results and patient approval. Our experience, built on a standardized and reproducible technique, allowed good surgical results in terms of operating times, prosthesis sizing, patients selection and clinical outcome, which could be considered at least comparable to those obtained with standard full sternotomy and would deserve being properly addressed in randomized comparative prospective trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the local Ethic committee (Comitato Etico Area Vasta Romagna CEIIAV with number 1189) and each patient signed an informed consent for the treatment of personal data.
References
- Rao PN, Kumar AS. Aortic valve replacement through right thoracotomy. Tex Heart Inst J 1993;20:307-8. [PubMed]
- Cosgrove DM 3rd, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996;62:596-7. [Crossref] [PubMed]
- Murtuza B, Pepper JR, Stanbridge RD, et al. Minimal access aortic valve replacement: is it worth it? Ann Thorac Surg 2008;85:1121-31. [Crossref] [PubMed]
- Tabata M, Umakanthan R, Cohn LH, et al. Early and late outcomes of 1000 minimally invasive aortic valve operations. Eur J Cardiothorac Surg 2008;33:537-41. [Crossref] [PubMed]
- Gilmanov D, Bevilacqua S, Murzi M, et al. Minimally invasive and conventional aortic valve replacement: a propensity score analysis. Ann Thorac Surg 2013;96:837-43. [Crossref] [PubMed]
- Plass A, Scheffel H, Alkadhi H, et al. Aortic valve replacement through a minimally invasive approach: preoperative planning, surgical technique, and outcome. Ann Thorac Surg 2009;88:1851-6. [Crossref] [PubMed]
- Glower DD, Desai BS, Hughes GC, et al. Aortic valve replacement via right minithoracotomy versus median sternotomy: a propensity score analysis. Innovations (Phila) 2014;9:75-81; discussion 81. [Crossref] [PubMed]
- Phan K, Xie A, Di Eusanio M, et al. A meta-analysis of minimally invasive versus conventional sternotomy for aortic valve replacement. Ann Thorac Surg 2014;98:1499-511. [Crossref] [PubMed]
- Ruttmann E, Gilhofer TS, Ulmer H, et al. Propensity score-matched analysis of aortic valve replacement by mini-thoracotomy. J Heart Valve Dis 2010;19:606-14. [PubMed]
- Glauber M, Miceli A, Gilmanov D, et al. Right anterior minithoracotomy versus conventional aortic valve replacement: a propensity score matched study. J Thorac Cardiovasc Surg 2013;145:1222-6. [Crossref] [PubMed]
- Sansone F, Punta G, Parisi F, et al. Right minithoracotomy versus full sternotomy for the aortic valve replacement: preliminary results. Heart Lung Circ 2012;21:169-73. [Crossref] [PubMed]
- Mikus E, Turci S, Calvi S, et al. Aortic valve replacement through right minithoracotomy: is it really biologically minimally invasive? Ann Thorac Surg 2015;99:826-30. [Crossref] [PubMed]
- Shehada SE, Öztürk Ö, Wottke M, et al. Propensity score analysis of outcomes following minimal access versus conventional aortic valve replacement. Eur J Cardiothorac Surg 2016;49:464-9; discussion 469-70. [Crossref] [PubMed]
- Shehada SE, Elhmidi Y, Mourad F, et al. Minimal access versus conventional aortic valve replacement: a meta-analysis of propensity-matched studies. Interact Cardiovasc Thorac Surg 2017;25:624-32. [Crossref] [PubMed]
- Schmitto JD, Mohr FW, Cohn LH. Minimally invasive aortic valve replacement: how does this perform in high-risk patients? Curr Opin Cardiol 2011;26:118-22. [Crossref] [PubMed]
- Ranucci M, Frigiola A, Menicanti L, et al. Aortic cross-clamp time, new prostheses, and outcome in aortic valve replacement. J Heart Valve Dis 2012;21:732-9. [PubMed]
- Murzi M, Cerillo AG, Bevilacqua S, et al. Traversing the learning curve in minimally invasive heart valve surgery: a cumulative analysis of an individual surgeon's experience with a right minithoracotomy approach for aortic valve replacement. Eur J Cardiothorac Surg 2012;41:1242-6. [Crossref] [PubMed]
- Glauber M, Miceli A, Bevilacqua S, et al. Minimally invasive aortic valve replacement via right anterior minithoracotomy: early outcomes and midterm follow-up. J Thorac Cardiovasc Surg 2011;142:1577-9. [Crossref] [PubMed]
- Grossi EA, Galloway AC, Ribakove GH, et al. Minimally invasive port access surgery reduces operative morbidity for valve replacement in the elderly. Heart Surg Forum 1999;2:212-5. [PubMed]
- Yamada T, Ochiai R, Takeda J, et al. Comparison of early postoperative quality of life in minimally invasive versus conventional valve surgery. J Anesth 2003;17:171-6. [Crossref] [PubMed]