Predicting outcome of venovenous ECMO: look outside the lung!
As a background, we acknowledge that the mortality rate of patients with severe acute respiratory distress syndrome (ARDS) is still elevated, exceeding 50% despite optimal supportive care (1-3) and that patients who survive have a worse quality of life in terms of functional, physical and psychological performances. Indeed, as long as we speculate in this setting, we assume to deal with patients who do not have life-threatening hypoxemia, as in this regard there would be no point against venovenous extracorporeal membrane oxygenation (VV ECMO). However, the human tolerability to hypoxemia is still a matter of debate and on clinical grounds we usually care for patients who have low P/F (i.e., 50 to 80) with protective ventilation and, eventually, ‘rescue maneuvers’. As a matter of fact, VV ECMO is a recognised life-saving therapy for patients with intractable refractory hypoxemia (primary ECMO) but there is still much discussion and controversy about the indications/contraindications of ECMO and the time of initiation, in patients who are failing on conventional treatment (secondary ECMO), especially as it is not straightforward ‘what is failure’ (4,5).
In this perspective, it is challenging to speculate on expected mortality and potential role of VV ECMO in reducing this outcome, especially as long as we consider this intervention valuable only in terms of restoring acceptable values of blood gases (6,7).
Notwithstanding that ARDS patients are a very heterogenous population, sometimes misdiagnosed, and comprising many different diseases and patients with various associated types and degrees of organ failure and comorbidities. Lastly, among the causes of death, sepsis and multiple organ failure remain the most common, while only a small percentage of deaths can be attributed to respiratory failure.
However, we believe that a new perspective should be put in place in the understanding and clinical management of these complex and heterogeneous patients, and to the role of VV ECMO. There is a complex interplay between the lung [primary pulmonary disorder and mechanical ventilation (MV)] and the heart (right and left ventricle) which, potentially, drives the precipitation into multiple organ failure; this is only partially related to hypoxemia or hypercapnia, and it affects the heart with different severity according to the baseline cardiac function and/or disease. In these patients, hypoxia and hypercapnia (acidosis) are responsible for pulmonary vasoconstriction; together with extrinsic vascular compression by interstitial edema and thromboembolic events these factors that contribute to increased PVR, pulmonary hypertension with right-ventricular afterload elevation, and eventually right-ventricular failure. Right-ventricular failure may further be worsened by the deleterious effect of inadequate MV. Acute cor pulmonale may cause or precipitate circulatory failure and its negative impact on outcome in patients with ARDS (8) has extensively described. Evaluating the right ventricle may help to open a new perspective to assess the equilibrium between recruitment and overdistension induced by the MV: indeed, the right ventricle appears as a modern determinant in tailoring the ventilatory strategy in patients with ARDS (9). Furthermore, ARDS is commonly associated with hemodynamic instability which can be related either to septic shock or to other causes. These pictures have the common feature of tachycardia, with or without hypercontractility, which is a driver for diastolic dysfunction with elevated filling pressures and pulmonary edema. These secondary mechanisms drive further deterioration of lung function, pulmonary hypertension and cardiogenic shock.
In this setting, VV ECMO allows ultraprotective MV and potentially less ventilator-induced lung injury.
Several papers (10,11) demonstrate how difficult it is to predict risk factors of mortality in patients with severe pulmonary failure who receive ECMO treatment; indeed, no consensus is available on futile treatments. Life-saving therapies cannot be studied in a randomized controlled trial (RCT), as the control group would be deprived of a chance of survival and no equipoise would jeopardize ethical approval.
Considering that ECMO implantation requires a specialized and well trained medical and paramedical staff, very high costs and requirements, and potentially complications, identifying the right timing and strategy for cannulation and stratifing the mortality risk factors is mandatory. On the other side, what is the key factor for being consistent on futility? As long as we do not encompass in this consideration patients who have preexisting chronic lung failure not candidate for transplantation, what is the downside of giving a chance to patients who would certainly fare bad? This approach is reasonable as long as there are no complications related to ECMO implantation, namely cannulation related. If this is so, what makes the difference with a third line chemotherapy to increase life expectancy of few months? Especially as we are treating acute illness, we should not speculate too much on prognostication beforehand rather proceed with cannulation. If we look at the risk scores which have been published up today, they reveal that extrapulmonary parameters are predictive of poor outcome: from our perspective, this stems from the assumption that VV ECMO implantation was deferred until strict respiratory criteria were met, and that was too late in the course of the disease. If we learn from these studies, we should move to a modern concept of indications and timing for VV ECMO, bearing in mind that patients’ severity is not simply measured by gas exchange and respiratory parameters. On the other side, once VV ECMO is timely started, it should facilitate to change the comprehensive management of the patient allowing for recovery of right ventricular function, weaning from vasoactive drugs and sedation, allowing for spontaneous breathing. If we endorse these concepts, we will make this technology a ‘milestone’ in the armamentarium of physicians treating acute lung failure and not a ‘rescue machine’.
As for background, the decision to institute VV ECMO is focused on the severity of pulmonary failure. In fact, according to the ELSO guidelines the use of ECMO should be considered when the oxygenation index (PaO2/FiO2 ratio) is <150, and ECMO is indicated when the ratio is <80. PaCO2 > 80 mmHg or plateau pressure >30 cmH2O is also considered an indication for ECMO in patients with ARDS (12).
Advanced age, a long duration of ventilation before ECMO, a higher Acute Physiology and Chronic Health Evaluation II (APACHE II) score, underlying lung disease, and pulmonary barotrauma prior to ECMO were associated with unsuccessful weaning from ECMO (13). In the last years, mortality risk models have been validated to predict outcome in this group of patients in order to help clinicians to select appropriate candidates for ECMO treatment: RESP-score (14) and PRESERVE-score (H), take into account lung and extra lung failure, Roch-score (15) and ECMO-net score (16) consider exclusively extrapulmonary organ function. The ECMO-net score (16), validated in 2013 by our group, concluded that mortality of adult patients suffering from influenza A (H1N1)-related ARDS undergoing VV ECMO was related to extrapulmonary organ function at the time of cannulation: preECMO hospital length of stay (days), bilirubin (mg/dL), creatinine (mg/dL), haematocrit (%), mean arterial pressure (mmHg), confirm author’s clinical perception that survival is strongly correlated to extra lung organ function at the time of ECMO initiation.
Recently the group of Essen-Germany validated the novel PRESET-Score (17) (Prediction of Survival Therapy) derived from the categorization of the five extrapulmonary variables independently associated with mortality measured immediately before ECMO initiation: admission pH, mean arterial pressure, lactate concentration, platelet count, and pre-ECMO hospital stay. The PRESET-Score gives a maximum of 15 points divided into 3 classes with an increasing mortality risk value. Risk class I: 0–5 points—mortality risk of 26%; risk class II: 6–9 points—mortality risk of 68%; risk class III: 10–15 points—mortality risk of 93%. See Table 1 for details.
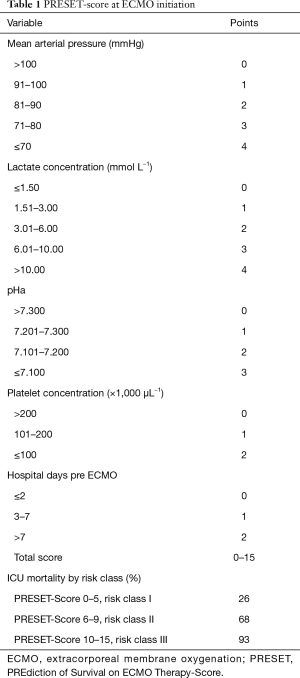
Full table
Again, this model doesn’t take into account any pulmonary variable, as they are not predictors of survival. Interestingly, the highest score does not reach enough power to define futility, and that raises ethical questions. At this point the debate is: which is the degree of extra lung dysfunction enable to predict outcome? If we acknowledge that in patients with ARDS blood gas and ventilatory parameters before ECMO are not self-sufficient to predict the final outcome, and these patients do not die from hypoxemia rather from multiorgan failure, and that is further confirmed by predictors of mortality in various risk models, we should identify extra pulmonary triggers for indications and timing of VV ECMO implant which not rely only on gas exchange. Following these considerations, the cited risk scores should maybe considered in the setting of ECMO implantation as “secondary therapy”, taking in mind that despite the usefulness of prediction models, the decision for ECMO should always be guided by a clinical evaluation. Moreover, once VV ECMO is implanted, management should follow these markers of severity and should be tailored accordingly.
This concept translates into a new vision in terms of general strategies around VV ECMO indications and general patient management.
The PRESET-Score, incorporating extrapulmonary variables, has a potential practical utility, predicts mortality better than previous scores, especially it is applicable also in limited resources settings (N). It is applicable to a broad range of ARDS patients, not discriminating influenza A (H1N1) patients, as previous scores (16).
As we have already emphasized, although this score is a useful prediction model, it should only supplement and facilitate the physician individual decision-making, based on patient conditions, history, and prognosis, therefore requiring experienced intensivists.
As an example, a 68 years patient with ARDS presenting with a PaO2 of 65 mmHg on FiO2 0.9, (PaO2/FiO2 72), respiratory rate 22/min, PEEP 13 cmH2O, mean airway pressure 27 cmH2O, but with circulatory failure (tachycardia 140 beats/min) CVP 17 mmHg and severe hypotension despite noradrenaline 0.5 mcg/kg/min) is more critical as compared to a patients more hypoxic (50 mmHg), PaO2/FiO2 50, respiratory rate 30/min, with higher mean airway pressure (37 cmH2O) and PEEP (18 cmH2O), but with stable hemodynamics (Table 2).
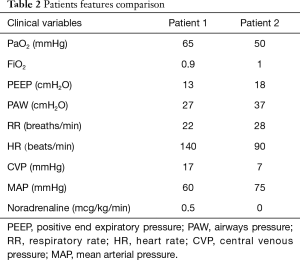
Full table
Concluding, the evaluation of ventilatory induced lung injury (VILI) should not only be limited to isolated lung injury but also to a much larger concept. The aggressive, detrimental and harmful ventilation usually performed as first step may worsen pulmonary function and irreversibly damage the lung, but the damage attributable to the mechanical ventilation is probably not limited to lung. In a different perspective, the decision to implant VV ECMO certainly depends on the degree of hypoxia (primary ECMO), but should also consider other criteria such as hemodynamic impairment, persistent need of sedation and controlled ventilation beyond 1 week, liver dysfunction and coagulation disorders, especially in older patients.
Acknowledgements
We are indebted to the San Raffaele Cardiac Intensive Care Nurse Staff for the everyday support on ECMO patients.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta- analysis. Intensive Care Med 2010;36:585-99. [Crossref] [PubMed]
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-08. [Crossref] [PubMed]
- Patroniti N, Zangrillo A, Pappalardo F, et al. The italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe 17. respiratory emergency outbreaks. Intensive Care Med 2011;37:1447-57. [Crossref] [PubMed]
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extractorporeal membrane oxygenation center and mortality among patients with severe influenza A(H1N1). JAMA 2011;306:1659-68. [Crossref] [PubMed]
- Combes A, Bacchetta M, Brodie D, et al. Extracorporeal membrane oxygenation for respiratory failure in adults. Curr Opin Crit Care 2012;18:99-104. [Crossref] [PubMed]
- Lee SH, Chung CH, Lee JW, et al. Factors predicting early and long-term survival in patients undergoing extracorporeal membrane oxygenation (ECMO). J Card Surg 2012;27:255-63. [Crossref] [PubMed]
- Enger T, Philipp A, Videm V, et al. Prediction of mortality in adult patients with severe acute lung failure receiving veno-venous extracorporeal membrane oxygenation: a prospective observational study. Critical Care 2014;18:R67. [Crossref] [PubMed]
- Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med 2013;39:1725-33. [Crossref] [PubMed]
- Repessé X, Charron C, Vieillard-Baron A. Acute cor pulmonale in ARDS: rationale for protecting the right ventricle. Chest 2015;147:259-65. [Crossref] [PubMed]
- Wagner K, Risnes I, Abdelnoor M, et al. Is it possible to predict outcome in pulmonary ECMO? analysis of pre-operative risk factors. Perfusion 2008;23:95-9. [Crossref] [PubMed]
- Schmidt M, Zogheib E, Roze H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013;39:1704-13. [Crossref] [PubMed]
- Extracorporeal Life Support Organization Guidelines. Available online: www.elso.org
- Liu X, Xu Y, Zhang R, et al. Survival Predictors for Severe ARDS Patients Treated with Extracorporeal Membrane Oxygenation: A Retrospective Study in China. PLoS One 2016;11:e0158061. [Crossref] [PubMed]
- Schmidt M, Bailey M, Sheldrake J, et al. Predicting Survival after Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) Score. Am J Respir Crit Care Med 2014;189:1374-82. [Crossref] [PubMed]
- Roch A, Hraiech S, Masson E. Outcome of acute respiratory distress syndrome patients treated with extracorporeal membrane oxygenation and brought to a referral center. Intensive Care Med 2014;40:74-83. [Crossref] [PubMed]
- Pappalardo F, Pieri M, Greco T, et al. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A(H1N1) pneumonia: the ECMOnet score. Intensive Care Med 2013;39:275-81. [Crossref] [PubMed]
- Hilder M, Herbstreit F, Adamzik M, et al. Comparison of mortality prediction models in acute respiratory distress syndrome undergoing extracorporeal membrane oxygenation and development of a novel prediction score: the PREdiction of Survival on ECMO Therapy-Score (PRESET-Score). Critical Care 2017;21:301. [Crossref] [PubMed]


