Circulating microRNAs as an emerging biomarker for acute aortic dissection diagnosis—comparing with prior biomarkers
Acute aortic dissection (AAD) is a serious disease with a high mortality rate. The mortality rate for patients with type A AAD who do not get treatment is of 1–2% per hour during the first 24 hours and almost 50% of the patients die within a week (1). The highest mortality associated with AAD occurs in the first 48 hours after onset of symptoms. Therefore, immediate diagnosis could be life-saving (2). However, symptoms of the disease and techniques used to detect the disease, such as chest radiography and electrocardiography (ECG), all lack sensitivity and specificity; therefore, diagnosis is not immediate (3). The International Registry of Acute Aortic Dissection (IRAD) reported that the sudden-onset and severe chest or back pain was the only and common presenting symptom, but the clinical symptoms of AAD were varied and about 10% of patients did not complain of pain. Classical physical examinations, such as pulse deficit and aortic regurgitation, were noted in only 15.1% and 31.6% of the patients, respectively. Furthermore, chest radiography and ECG were frequently not useful for diagnosis (abnormal findings were not detected in 12.4% and 31.3% of the patients, respectively) (1). Among patients with type A dissection, 12.7% of the patients exhibited syncope, and most did not show any other neurological findings. Therefore, dissection is often difficult to diagnose. Recently, a large medical center reported that 38% of consecutive patients with AAD were not diagnosed on initial assessment and 28% of the patients were first demonstrated during postmortem examination (4). Variables resulted in delayed diagnosis included female patients [hazard ratio (HR), 1.73], a normal blood pressure (HR, 2.45), transfer from a primary institution (HR, 3.3), and fever (HR, 5.1) (5). Computed tomography angiography (CTA) or echocardiography is usually needed for patients with clinically suspected AAD based on the symptoms and initial examinations. The previous studies reported that a mean sensitivity and specificity were more than 95% for both CTA, magnetic resonance imaging (MRI), and transesophageal echocardiography (6). These results suggest that all three imaging investigations yield clinically helpful results to diagnose or rule out AAD. In addition to supporting in the diagnosis of AAD, the findings of imaging can assist to manage AAD patients. Significant findings include the region of the dissection, size of the true and false lumen, involvement of aortic branches, localization of the intimal tear, presence of periaortic hematoma, presence and severity of aortic regurgitation, and pericardial effusion (7). However, performing each imaging investigation on patients with suspected AAD results in a delay in patient care. There are a number of biomarkers available that accelerate the diagnostic pathway, directing disease detection using imaging techniques and leading to definitive treatment (3). However, none of them are yet completely validated.
Recently, Dong et al. (8) reported that circulating microRNAs are novel potential biomarkers for diagnosing AAD. Between February 2011 and July 2012, a total of 63 aortic dissection (AD) patients and 23 non-AD patients who were hospitalized were analyzed in this study. AD was diagnosed by CTA. Of 63 AD patients, 37 patients were AAD, and 26 patients were subacute aortic dissection (SAD). Of 23 non-AD patients, 11 patients were acute myocardial infarction (AMI), two patients were pulmonary thromboembolism (PTE), and ten patients were aortic aneurysm (AA). Furthermore, 17 healthy controls were enrolled in the study. All participants without AD (n=40) were included in the control group. Of those 40 participants, 14 had chest pain (CP) (11 AMI, 2 PTE, and 1 AA patients). Blood samples to detect microRNAs were immediately obtained on arrival at hospital and were processed by two-step centrifugation within 1 hour after blood collection. The supernatant was stored at −80 °C, and total RNA was isolated from the plasma specimens by using miRNeasy Mini Kit (Qiagen, Hilden, Germany). The candidate microRNAs were identified by using Human MicroRNA Microarrays (Agilent Technologies, Santa Clara, CA, USA).
The expression levels of seven microRNAs in the AAD group, including miR-15a, miR-23a, let-7b, miR-663, miR-518e, miR-451, and miR-16, were significantly higher than those in the healthy group. On the other hand, plasma levels of eight microRNAs in the AAD group, including hcmv-miR-US33-5p, miR-3682, miR-3196, miR-3162, miR-3131, miR-1182, miR-192, miR-150, were significantly lower than those in the healthy group. The expression levels of miR-23a and miR-223 were higher in AAD patients than in AA patients. In summary, 16 candidate microRNAs with abnormal expression levels were identified for further analysis by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). Of those microRNAs, plasma level of miR-15a were significantly elevated in the AAD group compared to the control group (P=0.008). Receiver operating characteristic (ROC) analysis revealed an optimum cutoff level for miR-15a with a sensitivity of 75.7% and a specificity of 82.5% for AAD detection. The diagnostic accuracy of miR-15a determined by area under the curve (AUC) was 0.761. Based on clinical requirements, further investigation was done on whether plasma microRNA levels could be helpful in differentiating AAD from CP. Four microRNAs (miR-15a, miR-23a, let-7b, and US33-5p) were significantly elevated in the AAD group than in the CP group (P<0.0005, P=0.026, P<0.0005, and P=0.011, respectively). ROC analysis for these four microRNAs exhibited sensitivity of 75.7%, 91.9%, 79.4%, and 73.5%, respectively. The specificity values were 100%, 85.7%, 92.9%, and 85.7%, respectively. The corresponding AUCs were 0.855, 0.925, 0.887, and 0.815, respectively. In this study, the ROC analysis showed that microRNAs were more specific than the D-dimer for detection of AAD.
In conclusion, plasma level of miR-15a were significantly higher in the AAD group than in the control group (including healthy controls, AMI, PTE, and AA patients). Plasma level of miR-23a could be used for differentiating AAD from CP groups with high sensitivity and specificity. Both miR-15a and miR-23a have a long-time window and high accuracy for AAD detection. This study suggests that plasma miR-15a may be useful for screening and monitoring of AAD and plasma miR-23a is of potential clinical value for the differential diagnosis of AAD.
These results have important investigative implications. As AD occurs at medial layer, biomarkers reflecting injury to vascularized tissue had been researched, such as the vascular interstitium (calponin), vascular smooth muscle [smooth muscle myosin heavy chain (smMHC)], the elastic laminae [soluble elastin fragments (sELAF)], the extracellular matrix glycoprotein, which is highly up-regulated during inflammatory/healing response [tenascin-C (TN-C)] or the reactant secondary to exposure of blood to non-intimal vascularized tissue (D-dimer) (9). We collected data from previously published literature to investigate the availability of these biomarkers (Table 1).
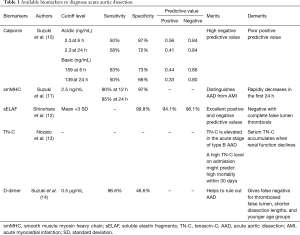
Full table
Suzuki et al. (10) demonstrated that circulating calponin levels were elevated in AAD. Calponin has three isoforms. For acidic calponin, optimum values of 2.3 ng/mL led to detection sensitivity of 50% and specificity of 87% during the first 6 hours, and sensitivity of 58% and specificity of 72% in the initial 24 hours. For basic calponin, optimum values of 159 ng/mL resulted in detection sensitivity of 63% and specificity of 73% during the first 6 hours, and optimum values of 139 ng/mL led to sensitivity of 50% and specificity of 66% in the initial 24 hours. For acidic calponin, positive and negative predictive values were 0.56 and 0.84 in the first 6 hours, and 0.41 and 0.84 in the initial 24 hours, respectively. For basic calponin, positive and negative predictive values were 0.44 and 0.86 in the first 6 hours, and 0.33 and 0.80 in the initial 24 hours, respectively. Calponin has acceptable negative predictive value in the initial 24 hours, but its positive predictive value is unsatisfactory. Suzuki et al. (11) reported that the sensitivity of smMHC was 90% and 85% at 12 and 24 hours, respectively, and the specificity was 97% at a cutoff level of 2.5 ng/mL. Its levels were not increased in patients with AMI. The serum level of smMHC was elevated in the first hour after onset of AAD, but rapidly decreased within the initial 24 hours. This suggests that smMHC could have availability if applied at early phase but its sensitivity rapidly decreases following symptom onset. Shinohara et al. (12) reported that serum level of sELAF might be an available marker for screening of AAD. Elevated expression levels of sELAF are sustained for up to 72 hours post-AAD. When the cutoff level for positive test result was set at the mean value +3 standard deviation (SD), the specificity was 99.8%. The positive predictive value was 94.1% and negative predictive value was 98.1%. Of the patients with either totally or partially open pseudolumen, 88.9% were positive for sELAF expression, while patients with an early occlusion of the pseudolumen did not exhibit sELAF positivity.
Nozato et al. (13) reported that TN-C level in serum was increased in patients who were during the acute phase of type B AD and higher TN-C levels at the time of admission might predict high mortality within 30 days. In addition, they demonstrated that high TN-C levels on a week after hospitalization might predict regression of the affected aorta. This demonstrate that high TN-C levels may provide a protecting effect for patients in chronic phase of AD.
Suzuki et al. (14) reported that D-dimer levels might be helpful to rule out AD. There were 96.6% of the sensitivity and 46.6% the specificity at a cutoff level of 0.5 µg/mL vs. controls. However, despite the excellent sensitivities associated with D-dimer estimation, false negative results are still possible in some patient subgroups. Hazui et al. (15) reported that D-dimer values are lower in younger patient group, and patients with shorter dissection length and thrombosed pseudolumen. Therefore, such patients could be wrongly diagnosed when D-dimer values were applied for AAD detection.
Presently, only D-dimer has a clinically reliable role in ruling out AD. However, Dong et al. (8) found that microRNAs were more specific than D-dimer to detect AAD. Their study showed a sharp increase in miR-15a plasma level in one AAD patient with bacteremia. Therefore, more research is needed to understand the role of microRNAs in patients with inflammatory disorders, such as infections, collagen diseases, and malignancies. In addition, while ROC analysis for microRNAs showed high sensitivity and specificity, it is important to understand which microRNAs are commercially available, whether they can be used easily, whether their assays are time consuming, and whether they are expensive or not. In the future, large clinical trials will provide further insights into the efficacy of microRNAs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet 2015;385:800-11. [Crossref] [PubMed]
- Ranasinghe AM, Bonser RS. Biomarkers in acute aortic dissection and other aortic syndromes. J Am Coll Cardiol 2010;56:1535-41. [Crossref] [PubMed]
- Spittell PC, Spittell JA Jr, Joyce JW, et al. Clinical features and differential diagnosis of aortic dissection: experience with 236 cases (1980 through 1990). Mayo Clin Proc 1993;68:642-51. [Crossref] [PubMed]
- Harris KM, Strauss CE, Eagle KA, et al. International Registry of Acute Aortic Dissection (IRAD) Investigators. Correlates of delayed recognition and treatment of acute type A aortic dissection: the International Registry of Acute Aortic Dissection (IRAD). Circulation 2011;124:1911-18. [Crossref] [PubMed]
- Shiga T, Wajima Z, Apfel CC, et al. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med 2006;166:1350-56. [Crossref] [PubMed]
- Golledge J, Eagle KA. Acute aortic dissection. Lancet 2008;372:55-66. [Crossref] [PubMed]
- Dong J, Bao J, Feng R, et al. Circulating microRNAs: a novel potential biomarker for diagnosing acute aortic dissection. Sci Rep 2017;7:12784. [Crossref] [PubMed]
- Mohamed SA, Misfeld M, Richardt D, et al. Identification of candidate biomarkers of acute aortic dissection. Recent Pat DNA Gene Seq 2008;2:61-5. [Crossref] [PubMed]
- Suzuki T, Distante A, Zizza A, et al. Preliminary experience with the smooth muscle troponin-like protein, calponin, as a novel biomarker for diagnosing acute aortic dissection. Eur Heart J 2008;29:1439-45. [Crossref] [PubMed]
- Suzuki T, Katoh H, Watanabe M, et al. Novel biochemical diagnostic method for aortic dissection. Results of a prospective study using an immunoassay of smooth muscle myosin heavy chain. Circulation 1996;93:1244-9. [Crossref] [PubMed]
- Shinohara T, Suzuki K, Okada M, et al. Soluble elastin fragments in serum are elevated in acute aortic dissection. Arterioscler Thromb Vasc Biol 2003;23:1839-44. [Crossref] [PubMed]
- Nozato T, Sato A, Hikita H, et al. Impact of serum tenascin-C on the aortic healing process during the chronic stage of type B acute aortic dissection. Int J Cardiol 2015;191:97-9. [Crossref] [PubMed]
- Suzuki T, Distante A, Zizza A, et al. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation 2009;119:2702-7. [Crossref] [PubMed]
- Hazui H, Nishimoto M, Hoshiga M, et al. Young adult patients with short dissection length and thrombosed false lumen without ulcer-like projections are liable to have false-negative results of D-dimer testing for acute aortic dissection based on a study of 113 cases. Circ J 2006;70:1598-601. [Crossref] [PubMed]


