A review of guidelines for lung cancer
Introduction
According to the National Academy of Sciences, clinical practice guidelines are “statements that include recommendations intended to optimize patient care” (1). Guidelines are informed by a systematic review of available evidence, and should assess the benefits and harms of each intervention, as well as of alternative options.
A large number of clinical practice guidelines are available in oncology, most of them produced by International or National scientific societies. Due to the extremely rapid evolution of this field of medicine, most guidelines need to be updated annually or, in some cases [e.g., U.S. National Comprehensive Cancer Network (NCCN)], even more frequently.
The relationship between cancer and host and, specifically, with the immune system has been an active field of investigation since many decades, leading to the introduction of commonly used approaches such as cytokines-based treatments in renal cell carcinoma (2) and cutaneous melanoma (3), or Bacillus Calmette-Guérin instillation for non-muscle-invasive bladder cancer after endoscopic resection (4). However, other immunologic strategies such as vaccination against cancer-specific antigens failed to show benefits in solid tumors (5) and, even if cytokines are still considered treatment options by most guidelines in specific types of tumors, their use has been largely reduced by the introduction of more effective and less toxic treatments (2).
More recently, the discovery that cancer cells can exploit some immune inhibitory receptors such as cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed death-1 (PD-1) and its ligands (PD-L1 and PD-L2) to escape immune system surveillance, led the way to the development of specific monoclonal antibodies. Following the results of clinical trials testing their efficacy, these molecules, known as immune checkpoint inhibitors, are being increasingly used for the treatment many solid tumors, including lung cancer.
In this paper we reviewed the recommendations about the use of immune checkpoint inhibitors in the latest version of four different lung cancer clinical practice guidelines, three made by Scientific National [American Society of Clinical Oncology (ASCO); Italian Association of Medical Oncology (AIOM)] and International [European Society of Medical Oncology (ESMO)] Societies and one by not-to-profit alliance of 27 leading American cancer centers (NCCN) (Table 1).
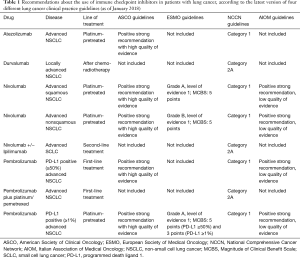
Full table
ASCO guidelines
The latest ASCO Guideline update on systemic therapy for stage IV non-small cell lung cancer (NSCLC) has been published online in August 2017 (6) and is based on the former version published in 2015 (7). This update was made using a “signals” approach that outlines formal criteria for identifying new, practice-changing data (8). By doing so, two major categories of changes were recognized as potential signals: potentially invalidating changes of evidence (opposing findings, evidence of substantial harm, evidence of superiority of a new treatment) and major changes in evidence (important changes in efficacy but not opposing findings, expansion of evidence of a treatment, important caveats). The expert panel reviewed the protocol used for the previous systematic review, then completed a formal literature review and sook for Expert Panel input and finally chose the updating option. Recommendations were developed, in part, using the Guidelines Into Decision Support (GLIDES) methodology (9) and accompanying BRIDGE-Wiz softwareTM. By doing so, recommendations were defined as evidence-based or based on either formal or informal consensus. The strength of each recommendation was rated as strong, moderate or weak, while the strength of evidence was rated as high, intermediate, low or insufficient. Finally the potential risk of bias was also rated.
As for first-line treatment, the panel considered the randomized phase III trial comparing pembrolizumab, a humanized monoclonal antibody against PD-1, vs. investigator’s choice of platinum-based chemotherapy, in patients who had previously untreated advanced NSCLC with PD-L1 expression on at least 50% of tumor cells, and no sensitizing mutation of the epidermal growth factor receptor (EGFR) gene or translocation of the anaplastic lymphoma kinase (ALK) gene (10). The primary endpoint of the trial was progression-free survival (PFS). Median PFS was 10.3 months in the pembrolizumab group versus 6.0 months in the chemotherapy group [hazard ratio (HR) 0.50; 95% confidence interval (95% CI), 0.37 to 0.68; P<0.001]. Pembrolizumab was also associated with a significant benefit in overall survival (HR 0.60; 95% CI, 0.41 to 0.89; P=0.005), in response rate (44.8% vs. 27.8%, with a longer duration of response), and in toxicity. Based on these results, ASCO guidelines suggest the use of single-agent pembrolizumab as first-line treatment in patients with advanced NSCLC, without EGFR activating mutations, ALK or ROS1 rearrangements and high PD-L1 expression (tumor proportion score-TPS ≥50%), in the absence of contraindications to immune checkpoint blockade. This recommendation is strong as it is evidence-based, with high quality of evidence.
In the second-line setting, recommendations are based on the randomized phase III trials comparing anti-PD-1 (nivolumab or pembrolizumab) or anti-PD-L1 (atezolizumab) monoclonal antibodies vs. docetaxel (11-14) in patients with advanced NSCLC who had previously failed first-line platinum-based chemotherapy. Trials of nivolumab and atezolizumab did not select patients according to PD-L1 expression, while trial of pembrolizumab was limited to patients with positive PD-L1 expression. In all those trials, primary endpoint was overall survival, and immune checkpoint inhibitors demonstrated a significant benefit compared to chemotherapy. Patients with EGFR mutation or ALK rearrangement were included in the trials, but subgroup analysis did not show a clear superiority for immune checkpoint inhibitors compared to chemotherapy (11-14). According to ASCO guidelines, the use of checkpoint inhibitors is suggested in NSCLC advanced patients without EGFR mutations or ALK and ROS1 rearrangements who did not receive pembrolizumab in the first-line setting. Coherently with inclusion criteria of the respective pivotal trials, patients with positive PD-L1 staining (TPS ≥1% with 22C3 assay) can be treated with either single-agent pembrolizumab, nivolumab or atezolizumab (strong evidence-based recommendation with high quality of evidence). Those with negative (TPS <1%) or unknown PD-L1 expression should receive nivolumab or atezolizumab monotherapies (strong evidence-based recommendation with high quality of evidence). The preferred second-line option for those treated with first-line pembrolizumab is standard platinum-based chemotherapy and, even if the quality of evidence is low (based on informal consensus among panelists, given the absence of trials specifically conducted in this setting), the recommendation is strong.
For patients with EGFR sensitizing mutations, already treated with specific tyrosine kinase inhibitors (TKIs) and platinum-based chemotherapy, the ASCO panel underlines that there are insufficient data to recommend immunotherapy in preference to chemotherapy (pemetrexed or docetaxel). This recommendation is weak and based on informal consensus among panelists as available evidence is insufficient, based on the small number of patients included in subgroup analyses.
In the immunotherapy field, the ASCO panel listed several issues suffering from lack of data and/or insufficient evidence: among those issues, contraindications to immune checkpoint inhibitors, their combinations with other checkpoint inhibitors or with chemotherapy, the treatment of patients who experienced toxicities during immunotherapy, the full utility of biomarker tests for PD-L1 expression.
The latest ASCO guideline on treatment of patients with small-cell lung cancer was published in 2015. Consequently, it does not contain any recommendation on immunotherapy.
ESMO guidelines
In 2017 ESMO published clinical practice guidelines for early stage and locally advanced NSCLC (15), while those on advanced NSCLC go back to 2016 (16) with an e-update in June 2017 (17).
ESMO guidelines are produced and updated by ESMO Guidelines Committee (GLC). Differently from other guidelines, these documents contain, beside thematic sessions, figures and algorithms, a personalized medicine synopsis table as well as a table with the ESMO-Magnitude of Clinical Benefit Score (MCBS) (18,19) for all the newly European Medicines Agency (EMA) approved therapies or indications.
ESMO MCBS is a dynamic tool developed to assess the magnitude of clinical benefit of new and effective cancer therapies. To reach this goal, a dual rule was implemented taking into account both the lower limit of the 95% CI for HR compared to a specified threshold value and the observed absolute difference in treatment outcomes for a given trial compared with the minimum absolute gain considered as beneficial. This scale is composed by two parts: part 1 evaluates treatments with curative intent (such as adjuvant chemotherapy) and is graded A, B or C where A and B represent the higher levels of clinical benefit; part 2 evaluates treatments without curative intent and is graded 5, 4, 3, 2, 1 where grades 5 and 4 represent the higher level of proven clinical benefit. MCBS calculations are performed by the Subject Editor who should involve the guidelines lead author to check and validate the scores produced. Moreover these scores are reviewed and approved by the guidelines steering committee, the MCBS Working Group and the ESMO President’s Council.
Recommendations are accompanied by proper level of evidence and grade of recommendation according to the adapted Infectious Disease Society of America-United States Public Health Service Grading System (20).
Guidelines on early stage and locally advanced NSCLC do not include any recommendation about immunotherapy, given that, when they were produced and published, results of the PACIFIC study on durvalumab after chemoradiotherapy in stage III NSCLC were not available yet (21). In the PACIFIC trial, patients with stage III NSCLC who did not have disease progression after platinum-based chemo-radiotherapy were randomized to durvalumab (anti-PD-L1 monoclonal antibody) or placebo. The co-primary endpoints were PFS and overall survival. Median PFS was 16.8 months with durvalumab versus 5.6 months with placebo (HR 0.52; 95% CI, 0.42 to 0.65; P<0.001). Durvalumab was also associated with a significant improvement in the response rate, in the duration of response and in the time to death or distant metastasis, while mature overall survival data are still not available.
Indeed, in the advanced disease setting, recommendations on immunotherapy are made for second-line treatment and beyond. In this setting, pembrolizumab at a dosage of 2 mg/kg every 3 weeks is considered a second- or third-line option (grade A recommendation, level of evidence I). As the phase III trial showed a superior outcome in patients with high PD-L1 expression (>50%), the ESMO-MCBS version 1.0 differs between those with PD-L1 >1% (score: 3) as compared to those with PD-L1 >50% (score: 5). Nivolumab is also recommended in platinum-pretreated patients in squamous histology (grade A, level I; ESMO-MCBS score: 5). However, in nonsquamous histologies, nivolumab is considered an option in pretreated patients (grade B, level I, ESMO-MCBS score: 5). Moreover, the panel underlines that, as it should be administered in second-line setting, only patients with PD-L1 positive tumors extract an OS benefit compared to docetaxel (grade B, level I), while those with PD-L1 negative tumors have similar OS with nivolumab and docetaxel, even if immunotherapy has a more favourable toxicity profile than chemotherapy (grade II, level A). No recommendations are provided on the use of pembrolizumab in the first-line setting.
ESMO Clinical Practice Guidelines on small cell lung cancer (SCLC) were published in 2013 and do not contain any recommendation on immunotherapy (22).
NCCN guidelines
As previously reported, NCCN Guidelines are evidence-based consensus-driven documents developed by a panel of members from 27 NCCN Member Institutions (23). Each guideline undergoes at least yearly an Institutional Review by clinical cancer experts from Member Institutions, even if interim Panel meetings are conducted throughout the year as needed. These guidelines are intended to assist all individuals who impact the decision making in cancer care also including payers, along with healthcare professionals and patients, given the fact that such documents are developed for an insurance-based national healthcare system.
NCCN categories of recommendations are based on both the level of clinical evidence available and the degree of consensus among the panel. The level of evidence depends upon three factors: extent of data, consistency of data, and quality of data. The degree of consensus is based on the percentage of panel votes. The cost of intervention is not formally considered even if, in some cases when robust data on pharmacoeconomics studies are available, panels may consider it.
NCCN categories are defined as: category 1, when based upon high-level evidence, with uniform consensus that the intervention is appropriate; category 2A, when based upon lower-level evidence but with still uniform consensus on appropriateness; category 2B, based upon the same level of evidence as the latter with NCCN consensus on appropriateness although not uniform; category 3 when, despite any level of evidence, there is major NCCN disagreement that the intervention is appropriate. In these guidelines, uniform consensus requires that at least 85% of Panel votes. The “NCCN consensus” required for category 2B is present when at least 50% (but less than 85%) of Panelists vote in favor of a recommendation. Finally, disagreement requires at least 3 Panel Members (from different Institutions) to vote.
The latest NCCN Clinical Practice Guideline on NSCLC has been published online on December 19th 2017 (24). Indications to immunotherapy are present both for the locally advanced setting and the metastatic one. The panel recommend as category 2A durvalumab (an anti-PD-L1 monoclonal antibody) as consolidation therapy for up to 12 months for patients with stage III NSCLC not progressing after definitive chemoradiation therapy. This recommendation is based on the results of the phase III PACIFIC trial (21). In the advanced setting the panel recommends nivolumab treatment as subsequent therapy in NSCLC patients progressing on or after first-line chemotherapy. This category 1 recommendation included both squamous and non-squamous histologies even if, in this latter group, the panel underlines the presence of a complementary diagnostic biomarker test assay that could help clinicians in choosing which patients may benefit most from immunotherapy. In this setting, NCCN Guidelines recommend also pembrolizumab (in patients with NSCLC and PD-L1 expression levels of 1% or more—category 1) or atezolizumab, independently of PD-L1 expression (category 1).
When treating patients in the first-line setting, NCCN recommends pembrolizumab treatment in patients whose tumors show high PD-L1 expression (more or equal to 50%) and without oncogene addiction (category 1). In this view, the panel explicitly recommends immunohistochemistry (IHC) testing of PD-L1 before first-line treatment in all patients with advanced NSCLC with negative or unknown tests results for known oncogenes (category 2A).
For patients with advanced untreated non-squamous or not otherwise specified (NOS) NSCLC, NCCN guidelines recommend combination treatment with pembrolizumab plus carboplatin plus pemetrexed, based on the results of a cohort of a randomized phase 2 trial that led to the FDA approval of this combination (category 2A) (25). Notably, for this indication, patients need not to be selected on the basis of PD-L1 expression levels.
NCCN guidelines on SCLC have been published, in their latest version, in September 2017 (26).
The panel recommends second-line therapy with nivolumab or nivolumab plus ipilimumab in patients with SCLC relapsed 6 months or less after primary treatment as category 2A. This recommendation is based on the results of a phase I/II trial (27).
AIOM guidelines
The Italian Association of Medical Oncology (AIOM) guidelines are made by using an adapted GRADE methodology (28). Every year, synchronously with AIOM annual meeting, guidelines are updated and published on the society website. Every recommendation is based on a clinical question formulated using PICO (population, intervention, control, and outcomes). Recommendations are graded on a four levels scale: strong positive, weak positive, weak negative, and strong negative. Quality of evidence is judged as high, moderate, weak, or very weak.
Notably, these documents contain recommendations that could be implemented in the national context and, by doing so, encompass only interventions that have been approved for use in clinical practice by Italian authorities and reimbursed by National Health System. The latest version of AIOM lung cancer guidelines has been published in October 2017 (29).
With regard to immunotherapy, the panel recommendation about the use of pembrolizumab as first-line monotherapy in patients with advanced NSCLC and high PD-L1 expression (≥50%) is strong, even if the quality of the evidence is considered low. Indeed, the authors underline potential bias on the Keynote-024 trial such as the lack of blinding, the sponsor participation in trial design as well as in data analysis and manuscript writing, and the early study interruption (even if based on pre-specified interim analysis).
In the second-line setting, nivolumab as well as pembrolizumab (in patients with ≥1% of PD-L1 positive cells) should be considered the first treatment option in both squamous and non-squamous NSCLC patients. To date, atezolizumab is approved by European Medicines Agency, but it is still not reimbursed for use in clinical practice in Italy. The panel evaluated the global quality of evidence in this context as low, but strongly recommends this intervention as compared to docetaxel chemotherapy.
No recommendations on immunotherapy and SCLC are present, as checkpoint inhibitors are not registered with this indication neither in Europe nor in Italy.
Discussion
Clinical practice guidelines represent an essential tool that helps clinicians in the decision making process, especially in rapidly evolving fields, such as oncology. In this context, different international as well as national scientific oncology societies produce documents with the aim of implementing cancer care and distributing knowledge among physicians as well as health care professionals.
The crucial role of updated guidelines is particularly evident when innovative interventions, such as immune checkpoint inhibitors in lung cancer, are made available. As all novelties could bring, along with advances in care, unresolved issues or debated questions on their appropriate use, guidelines have the important role of widely harmonizing their adoption.
Because guidelines greatly differ in methodology, formal comparisons between them are incorrect. However, strengths and weaknesses of different documents deserve a discussion.
ASCO guidelines are made using a well-described methodology making them, in principle, a robust tool for clinical decision making. However, they lack a continuous or time-predefined updating, and, at least at some time points, their applicability in this rapid evolving field is substantially limited.
ESMO guidelines suffer of the same limitations of ASCO ones, even if online updates are more frequent. Although ESMO members have recently increased also outside Europe, ESMO documents are intended to be used in the European countries and for this reason they include EMA-approved interventions only. Of note, by using the ESMO MCBS, they include an evaluation of the value of each intervention. Even if this tool should be viewed as an effort to introduce some elements from the public health perspective in an otherwise purely clinical document, there are some great limitations that need to be underlined. As already reported by Sobrero and colleagues, this scale adopts a mono-dimensional, protocol-driven approach, focused on the evaluation of single randomized trial results, thus missing the complex, multidimensional and sometimes indirect evidence available for many drugs (30). Other limitations of the first ESMO MCBS version include the use of fixed HR thresholds for classifying drugs (favoring small trials with more variable HR estimates) and the lack of toxicity-driven downgrading of the score when an OS benefit is observed. As the clinical benefit is defined by the integration of efficacy, toxicity and convenience, the ESMO MCBS lack the latter two dimensions by focusing on efficacy only. However, since immune checkpoint inhibitors always demonstrated a favorable toxicity profile as compared to chemotherapy in all available randomized trial, we acknowledge that the issue on toxicity-driven downgrading would not be an issue in this context. However, some of the above cited limitations and weaknesses have been addressed in the revised version (1.1) of the ESMO MCBS (19).
NCCN guidelines carry the great advantage of being updated every time a new intervention becomes available in the United States following the FDA approval. This is critical in an insurance-based health system such as that of the US where central drugs approval is extremely fast by limiting the need of price and reimbursement discussions. For this reason, these guidelines are very useful in providing all the possible options for a given clinical situation especially when taking into account novel drugs. However they are weaker in providing comparative tools to help clinicians in clinical decision making and they lack a strong methodologic background.
AIOM guidelines, on the contrary, are made with a robust methodology. They are annually updated and contain recommendations on all the available options for a given clinical question in the Italian context. For this latter reason, their transferability beyond the Italian borders is the major limitation.
Bearing in mind all these caveats and limitations, as a general rule, all clinicians should make their best in studying and applying clinical practice guidelines, because their use would substantially improve homogeneity and appropriateness of interventions adopted in clinical practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: P Bironzo—Honoraria: Bristol Myers Squibb; Research funding: Merck Sharp & Dohme. M Di Maio—Honoraria: Bristol Myers Squibb, Merck Sharp & Dohme, Eli Lilly, AstraZeneca, Janssen; Consulting or advisory role: Janssen, AstraZeneca, Eli Lilly; Research funding: Tesaro.
References
- Graham R, Mancher M, Wolfman DM, et al. editors. Clinical Practice Guidelines We Can Trust. Washington: The National Academies Press, 2011.
- Escudier B, Porta C, Schmidinger M, et al. Renal Cell Carcinoma: ESMO Clinical Practice Guidelines. Ann Oncol 2016;27:v58-68. [Crossref] [PubMed]
- Dummer R, Hauschild A, Lindenblatt N, et al. Cutaneous Melanoma: ESMO Clinical Practice Guidelines. Ann Oncol 2015;26:v126-32. [Crossref] [PubMed]
- Bellmunt J, Orsola A, Leow JJ, et al. Bladder Cancer: ESMO Clinical Practice Guidelines. Ann Oncol 2014;25:iii40-8. [Crossref] [PubMed]
- Vansteenkiste JF, Cho BC, Vanakesa T, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2016;17:822-35. [Crossref] [PubMed]
- Hanna N, Johnson D, Termin S, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3484-515. [Crossref] [PubMed]
- Masters GA, Termin S, Azzoli CG, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2015;33:3488-515. [Crossref] [PubMed]
- ASCO Guidelines. Methodology supplement. Available online: (Last accessed on February 25, 2018).http://ascopubs.org/doi/suppl/10.1200/JCO.2017.74.6065/suppl_file/ms_2017.74.6065.pdf
- GuideLines Into DEcision Support (GLIDES). Available online: (Last accessed on February 25, 2018).http://medicine.yale.edu/cmi/glides/
- Reck M, Rodrìguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1 positive non-small-cell lung cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel in previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early-Stage and Locally Advanced (non-metastatic) Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guidelines. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1-v27. [Crossref] [PubMed]
- eUpdate – Metastatic Non-small-cell Lung Cancer: Treatment Recommendations and Revised ESMO Magnitude of Clinical Benefit Scale (ESMO-MCBS) Grading. Available online: (Last accessed on February 25, 2018).http://www.esmo.org/Guidelines/Lung-and-Chest-Tumours/Metastatic-Non-Small-Cell-Lung-Cancer/eUpdate-Treatment-Recommendation
- Cherny NI, Sullivan R, Dafni U, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society of Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol 2015;26:1547-73. [Crossref] [PubMed]
- Cherny NI, Dafni U, Bogaerts J, et al. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol 2017;28:2340-66. [Crossref] [PubMed]
- Infectious Diseases Society of America. Guideline Methodology and Other Resources. Available online: (last accessed on January 13th, 2018).http://www.idsociety.org/uploadedFiles/IDSA/Guidelines-Patient_Care/Guidelines_By_Others/IDSA%20Handbook%20on%20CPG%20Development%2010.15.pdf
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Früh M, De Ruysscher D, Popat S, et al. Small-Cell Lung Cancer: ESMO Clinical Practice Guidelines. Ann Oncol 2013;24:vi99-vi105. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Development and Update of the NCCN Guidelines. Available online: (Last accessed on January 14th, 2018).https://www.nccn.org/professionals/development.aspx
- NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer. Available online: (Last accessed on January 14th, 2018).https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Small Cell Lung Cancer. Available online: (Last accessed on January 17th, 2018).https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf
- Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Linee guida AIOM. Available online: http://www.aiom.it/professionisti/documenti-scientifici/linee-guida/1,413,1, (Last accessed on January 20th, 2018).
- Linee guida AIOM. Neoplasie del polmone. Available online: (Last accessed on January 20th, 2018).http://www.aiom.it/professionisti/documenti-scientifici/linee-guida/1,413,1,#TopList
- Sobrero A, Puccini A, Bregni G, Bruzzi P. The urgent need to improve the tools to assess clinical benefit and value of cancer treatment. Eur J Cancer 2017;83:324-8. [Crossref] [PubMed]


