Pneumatosis intestinalis in solid organ transplant recipients
Introduction
Pneumatosis intestinalis (PI) is gastrointestinal (GI) condition characterized by the presence of gas within the bowel wall (1). PI was first reported by Du Vernoi in 1730 during cadaveric dissection (2), and has since been described in various clinical settings, including premature infants with necrotizing enterocolitis, adults diagnosed with pulmonary disorders, and immunosuppressed patients, particularly organ transplant recipients and patients diagnosed with HIV/AIDS. As the clinical settings in which PI has been described vary greatly, so too do its clinical presentations, ranging from asymptomatic, incidental detection on radiography to presentations associated with pneumoperitoneum and abdominal catastrophes (e.g., massive GI bleeding and intestinal ischemia) (3,4).
PI associated with solid organ transplantation (SOT) poses a unique clinical challenge, as SOT recipients are not ideal candidates for further surgical intervention. Over the past several decades, many have reported benign clinical presentation of PI in SOT recipients. These reports generally advocate a conservative approach in PI management; however, no standard treatment protocols or practice guidelines are currently available to aid clinicians in these clinical scenarios. In a 1990 review, Andorsky et al. (5) suggested that PI was frequently a benign, self-limited process. However, that review lacked details on the management and surveillance of patients diagnosed with PI. The incidence, diagnosis, and management of PI in SOT recipients must be better elucidated.
Methodology
We carried out a MEDLINE search using the following MeSH terms: “pneumatosis intestinalis”, “solid organ transplant”, “lung transplant”, “heart transplant”, “heart-lung transplant”, “kidney transplant”, and “liver transplant”. Clinical information extracted from these studies included patient demographics, presenting symptoms, time of symptom onset from the time of transplantation, immunosuppressive treatment modality, and outcomes. Analysis of these variables is presented in a descriptive format, as the relatively small number of patients and the inconsistency of data reported preclude statistical analysis. We have grouped our findings based on the organ transplanted—kidney, liver, heart, and lung.
Results
In total, we analyzed 37 articles published between 1979 and 2015 that described PI in various settings. We included studies and case reports describing PI in recipients of kidney, liver, heart, or lung transplant, where PI was discovered either in the perioperative course or in the late post-transplant course. Twelve of these articles described bone marrow transplant patients; these articles were excluded from study. This yielded nine articles on PI after kidney transplant, eight articles on PI after liver transplant, and eight articles on PI after cardiothoracic transplant (heart, lung, or both).
Kidney transplant recipients
Reports of PI in kidney transplant recipients are fairly sparse compared with reports of PI in other SOT recipients. All patients presented within 2 years of transplant: 71% of patients within 2 months of transplant; 93% of patients within 6 months of transplant. Most patients presented with either no symptoms at all or only with vague, mild symptoms such as abdominal pain, nausea/vomiting, and diarrhea. Of the 14 patients described in these articles, 13 proceeded with conservative management of their PI and completely recovered. One patient died, secondary to significant upper GI bleeding (Table 1). Of note, this patient was also the only one observed to have PI of the upper GI tract (8).

Full table
van Son et al. presented the first case series in 1985 (8), detailing outcomes of four patients diagnosed with PI after kidney transplant. All four patients had concurrent severe cytomegalovirus infection at the time of PI diagnosis. Two patients had no symptoms, one had mild abdominal pain, and one patient had severe GI bleeding. In one patient, the diagnosis was made retrospectively; this patient had no symptoms and made a full recovery with no intervention. Two patients were treated with oxygen via a Ventimask (Flexicare Medical Limited, Irvine, California), and both demonstrated quick resolution of PI. The remaining patient died after complications related to severe GI bleeding. The report made no mention of exploratory laparotomy in this patient. One year later, Ammons et al. described clinical improvement in three patients whose PI was managed conservatively (9). Two of the three patients were asymptomatic; their PI was discovered incidentally on abdominal radiography performed for other indications. The third patient presented with mild abdominal pain and diarrhea. All three patients were managed with clear liquid diets, and two showed complete resolution within 2 weeks. The third patient remained asymptomatic but PI was persistent, albeit decreased, 6 weeks later.
The final case series of PI in kidney transplant recipients was compiled by Murphy (10). Of the three patients in this report, the first presented with a severe, disseminated herpes infection. He developed right colon distention, prompting abdominal radiography and resulting in the diagnosis of PI. This patient’s PI resolved in three weeks, and no mention was made of any special therapy. The second patient similarly presented with mild abdominal pain and right colon distention that also resolved with conservative measures. PI in the third patient was discovered incidentally and remained completely asymptomatic, resolving within 8 weeks with a non-operative approach.
Liver transplant recipients
The first mention of PI in liver transplant patients was by Koep and colleagues in 1979 in a review on the diverse colorectal complications associated with liver transplantation (Table 2) (4). Eight patients were described in this review; two of these were diagnosed with PI, whereas the other six patients had different diagnoses, such as bowel perforation. In the two patients diagnosed with PI, the PI was discovered during laparotomy for life-threatening GI bleeding that occurred in the postoperative period. The authors concluded that surgery was necessary when there was massive bleeding associated with PI. Koep reflected: “Conservative therapy has been effective when pneumatosis cystoides intestinalis is identified roentgenographically. However, when [PI] is associated with sepsis or bleeding this suggests further disruption of mucosal integrity [and] resection is necessary” (4).

Full table
Several cases of postoperative PI were reported in liver transplant recipients in the late 1980s (15,16). Janssen and colleagues described a 5-year-old boy who underwent two successive liver transplants after the first was chronically rejected, who presented three months after his second liver transplant with colicky abdominal pain. He had severe scrotal swelling, and was taken to the operating room for inguinal exploration, at which time he was found to have colonic pneumatosis. Postoperatively, he was treated with supplemental oxygen and metronidazole. Follow-up imaging showed regression and eventual resolution of the PI 4 months later.
Sachse and colleagues reported a case of postoperative PI in a 5-year-old girl who had undergone liver transplant (16). Her postoperative course was complicated by rejection episodes resistant to bolus pulse-dose corticosteroids. She developed nausea and vomiting 14 months post-transplant, and PI was diagnosed based on findings of abdominal radiography. The patient was initially managed with bowel rest except for immunosuppressive medications (cyclosporine and methylprednisolone) and trimethoprim-sulfamethoxazole. She was discharged home but returned with nausea, vomiting, and distention. She was then treated with imipenem-cilastatin, kanamycin, nasogastric decompression, and an elemental diet for 1 week. Although there was evidence of resolution of the PI within several days, the patient died 20 months after transplant from ongoing progressive rejection.
In 1992, King and colleagues described two pediatric liver transplant recipients diagnosed with PI, identified on days 7 and 8 postoperatively (17). The second patient experienced a short episode of rejection which was treated with increased steroids. This increased dose of steroids was initiated before PI was discovered. Air was identified in the portal vein on routine ultrasonography in both patients, and subsequent abdominal radiographs confirmed the presence of PI. Neither patient was managed differently, as they both remained asymptomatic with benign abdominal examinations without any signs of end-stage organ damage. The authors posited that benign PI in such clinical settings does not warrant further therapy. They also described the advantage of using ultrasonography to detect portal venous gas that may be detected on ultrasound in the context of a normal radiograph.
The two largest studies on PI in liver transplant recipients were authored by Kwon et al. and Burress et al. (18,20). In 1996, Burress detailed the clinical courses of six liver transplant recipients with post-transplant PI. In their study, four of six patients had at least one recent episode of acute rejection before PI was diagnosed; however, all four of these patients were more than 2 weeks out from any steroid pulses when the PI was identified. All six patients had complete resolution of PI with nonsurgical management. Treatments varied somewhat between antibiotic regimens used, duration of these regimens, and patients’ dietary status. One patient died 5 months after transplant while undergoing esophageal reconstruction surgery for an indication unrelated to his prior diagnosis of PI.
One of the largest and most recent retrospective series describing PI in liver transplant recipients was written by Kwon and colleagues (20). They presented the different computed tomography (CT) imaging characteristics in 22 patients diagnosed with PI along with descriptions of the clinical status of each patient. 81% of patients with findings of PI after liver transplant had complete resolution of PI; four patients in their study died. Associated clinical findings in patients with high mortality included hypotension, fever, or both—findings that were not seen in patients whose symptoms resolved. CT imaging in the group with high mortality showed severe findings, such as evidence of organ infarction and hemorrhagic ascites in addition to PI (20).
Cardiothoracic (lung and heart) transplant recipients (Figures 1-4)
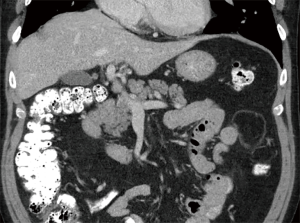
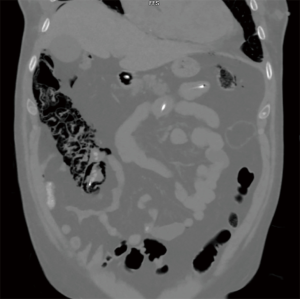
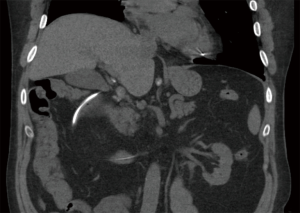
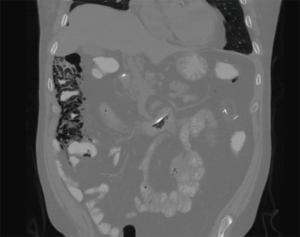
In 1990, Andorsky and colleagues presented the first case of PI after heart transplant (Table 3) (5). A 45-year-old heart transplant recipient presented with abdominal distention 4 months after transplant and was found to have both PI and pneumoperitoneum on radiography and on CT. A colonoscopy confirmed the presence of PI. This patient was managed with oxygen therapy for 24 hours and was discharged without incident (5). Fleenor and colleagues described eight pediatric patients who presented with PI after heart, lung, or heart–lung transplant (22). Time to symptom onset varied from 3 weeks to 36 months (average, 10 months). Symptom type also varied, from no symptoms to emesis, bloody diarrhea, or abdominal pain. Several patients had concurrent Clostridium difficile infection or rotavirus infection at the time of diagnosis. Patients with PI were significantly younger than those without (2.8 vs. 6.2 years, P=0.4). It was also noted that patients who developed PI were taking higher doses of steroids than patients who did not. The authors also noted that no patient whose steroid dose was <0.4 mg/kg/d developed PI. Higher serum tacrolimus levels were also reported as a significant risk factors for PI. All patients with PI were treated with intravenous antibiotics, were kept non per os (NPO), and were started on total parenteral nutrition (TPN) for 10–21 days. One of the eight patients had an examination suggestive of perforation, which prompted exploratory laparotomy; however, the findings of that procedure were not discussed in the paper. There were no related deaths (22).
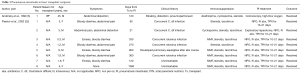
Full table
Thompson and colleagues most recently published their experience with seven patients who underwent bilateral lung transplant and developed PI, pneumoperitoneum, or both (Table 4). The mean time to development of PI/pneumoperitoneum from transplant was 6 months. Most patients were asymptomatic or had mild abdominal pain that was sometimes associated with nausea, vomiting, or both. One of the seven patients underwent exploratory laparotomy for potential intestinal perforation and ischemia. This patient had normal laboratory studies and only mild changes in her abdominal examination. During the exploratory laparotomy, she was found to have extensive colonic pneumatosis but no concerning pathology. All other patients were treated non-operatively and were kept NPO with serial abdominal examinations for the next 72 hours. Regular feedings were resumed on an average of 5 days after findings of PI. The mean time to radiographic resolution of PI was 23 days, though not all patients were followed with serial imaging until resolution (26).
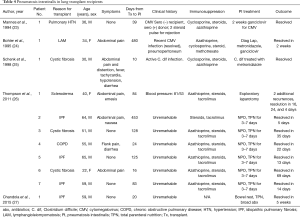
Full table
Discussion
Despite being well documented over the past 30 years, PI remains a somewhat enigmatic finding. In addition to being seen in SOT recipients, PI has been associated with over 50 other medical conditions (28). The true incidence of PI is unknown, as it is frequently discovered incidentally in asymptomatic patients. Several theories exist to explain the pathophysiology of PI, including mechanical, bacterial, infectious, and atrophy of GI lymphoid aggregates origins (1,22).
The mechanical theory indicates two possible causes of PI. The first involves elevated intraluminal pressures with subsequent infiltration of bowel gas through the mucosa. PI that occurs in patients post-endoscopy or in patients with bowel obstruction, volvulus, or constipation supports this aspect of the theory. The second aspect of this theory is that patients with chronic obstructive pulmonary disorder or a similar airway disease that results in alveolar rupture may be at a higher risk for PI secondary to gas dissection. Air could dissect down through the mediastinum and track along the aorta and mesenteric vessels, leading to PI formation.
The bacterial or infectious theory for PI formation suggests that gas produced by GI flora is responsible for PI. Studies of the gaseous composition of PI cysts have shown elevated levels of hydrogen, a byproduct of bacterial metabolism. Animal studies from the early 1970s of Clostridium injections in the bowel wall resulted in PI formation, which supports this theory (29). The final theory, albeit less discussed, is the theory of atrophy of GI lymphoid aggregate. Proponents of this theory suggest that some sort of immunologic suppression, be it serious disease, chemotherapy, or immunosuppressive medications used in certain patient groups (e.g., SOT recipients) is responsible for PI. Histologic studies have shown that GI lymphoid aggregates in some patients diagnosed with PI appear atrophic. These compromised areas of the intestinal mucosa could serve as a route for intraluminal gases to penetrate the bowel wall (30). This theory is supported by evaluation of PI incidences in SOT, such as those reviewed in this paper, in which nearly all patients were on high doses of steroids and other immunosuppressants.
In this review, we found that the majority of SOT recipients who develop PI had no symptoms at all or had only minimal, mild symptoms. The most common symptom was abdominal pain, although the severity and location were not always documented in the articles we reviewed. Other common symptoms included nausea, vomiting, and diarrhea. Fever and abdominal distention were also noted in several patients. GI bleeding, usually in the form of bloody diarrhea, was observed in some cases, but almost exclusively in pediatric and neonatal patients.
PI is diagnosed overwhelmingly by abdominal CT in the more recent papers reviewed, and by radiography in older literature (Figures 1-4). It is occasionally first diagnosed during colonoscopy, with subsequent CT or radiography used to confirm and evaluate the extent of disease. In many asymptomatic patients, PI is found incidentally on imaging obtained for other reasons. Once PI is diagnosed, the challenge becomes determining whether its presence warrants intervention. Over the past 20 years, several authors have proposed treatment algorithms for the management of PI. In the most recent of these by Tahiri et al. (31), several risk factors may prompt surgical exploration of the abdomen, even if the patient is stable. Informed by the 2013 Pneumatosis Intestinalis Predictive Evaluation Study, risk factors include lactic acid greater than 2.0 mmol/L, pH less than 7.3, bicarbonate level less than 20 mEq/L, and amylase level greater than 200 U/L (31,32). However, none of the cases presented in this review made mention of using these laboratory values.
Effective treatment is perhaps the most disputed topic when it comes to PI in SOT recipients. Numerous treatment strategies have been attempted over the past three decades, none of which have been studied enough to demonstrate superiority to any other modality. Treatment strategies were examined from the studies obtained in this review. Unfortunately, few of these studies mentioned the specific treatment method(s) used for these patients. Strategies discussed included operative exploration, NPO status, TPN, elemental diet supplementation, empiric antibiotics, hyperbaric oxygen, and serial abdominal examinations. Perhaps the most notable finding in this review is that only 3 of the 64 patients reviewed (4.7%) required operative exploration. The vast majority of patients were managed non-operatively (conservatively) and their symptoms resolved over time. Some authors mentioned patients who were successfully managed only with observation and without any type of intervention. The two patients mentioned in the study by King et al. were kept on their current diet without use of antibiotics (17). Conservative management seems to be the treatment modality of choice when PI is diagnosed in patients with a benign presentation.
There are several notable limitations in this review. Primarily, the data presented from these articles lacks uniformity. All articles covering this topic included data regarding transplanted organ, age, gender, presenting symptoms, and time of onset. However, clinical findings such as laboratory values, vitals upon admission, physical examination, imaging studies, treatment strategy, immunosuppression, and resolution of PI were inconsistently reported. Variability was also found in the management of immunosuppressive agents and whether doses were increased, decreased, or unchanged in the time surrounding the diagnosis of PI. Descriptive treatment details varied across studies, ranging from exact antibiotic dosing, timing, and follow-up schedules, to making no mention of treatment specifics at all. Furthermore, the definition of “resolution of PI” was inconsistent among studies. Some determined PI resolution radiographically, as followed by serial imaging. Others made no mention of repeat imaging. This lack of consistency made it difficult to conclude which management strategies were most effective.
Conclusions
PI presents a unique but challenging situation for clinicians, as the severity of illness is notoriously difficult to assess. This review examines the literature regarding PI and SOT over the last 40 years. The last similar comprehensive review was compiled over 25 years ago by Andorsky et al. (5). Patients described in the current review presented with PI during the postoperative course after transplant and their clinical presentation and management were highly variable. Many studies have described patients with PI in the general population who have underlying bowel ischemia, peritonitis, or massive bleeding. Therefore, the possible need for operative intervention is routinely taken into account in every situation. In the immunosuppressed SOT population, the risks associated with exploratory surgery are inherently higher, given issues with prolonged recovery and poor wound healing. Non-operative management should be considered when the clinical presentation of PI is not associated with clinical and laboratory indications to suggest abdominal catastrophes. Although there is no consensus regarding the specific conservative management strategies; bowel rest with NPO status, empiric antibiotics, serial abdominal examinations, parenteral nutrition and elemental feeding are commonly considered.
The data gathered from these studies show that no consensus exists for situations requiring operative management; timing, duration, or type of antibiotics used; and timing, duration, or type of diet modification selected (e.g., NPO status, tube feeding, and TPN supplementation). Further studies are needed to assess these variables independently. Given the reports that these patients can be managed without any change in treatment as seen in the study by King et al. in 1992 (17), nonoperative measures should be considered primarily unless there is a high index of suspicion for underlying peritonitis.
Future prospective studies are needed to examine the specific nonoperative (conservative) management principles in these patients. Patients could be divided into clinically unstable or septic and asymptomatic cohorts. Once nonoperative measures are deemed appropriate, these patients could be randomized to any combination of treatment measures including no change in management, antibiotic use, diet modification, and possibly oxygen therapy. Resolution of PI could be assessed simply with serial radiographs and abdominal exams. This would help practitioners reach a consensus on the ideal nonoperative treatment strategy to implement in these challenging patients. Ultimately, unnecessary treatment modalities could be abandoned and save patients from the time, expense, and potential downstream consequences of excessive antibiotic use, extended hospital stays, and NPO status. We suspect that PI in the SOT population is often clinically insignificant, and is likely related to use of immunosuppressive agents.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Heng Y, Schuffler MD, Haggitt RC, et al. Pneumatosis intestinalis: a review. Am J Gastroenterol 1995;90:1747-58. [PubMed]
- Du Vernoi JG. Anatomische Beobachtung der unter der auberen und innerin Haut der Gedarme eingeschlossenen. Luft Phys Med Abhandl Acad Wissensch Petersb 1783;2:182.
- Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. AJR Am J Roentgenol 2007;188:1604-13. [Crossref] [PubMed]
- Koep LJ, Peters TG, Starzl TE. Major colonic complications of hepatic transplantation. Dis Colon Rectum 1979;22:218-20. [Crossref] [PubMed]
- Andorsky RI. Pneumatosis cystoides intestinalis after organ transplantation. Am J Gastroenterol 1990;85:189-94. [PubMed]
- Julien PJ, Goldberg HI, Margulis AR, et al. Gastrointestinal complications following renal transplantation. Radiology 1975;117:37-43. [Crossref] [PubMed]
- Polinsky MS, Wolfson BJ, Gruskin AB, et al. Development of pneumatosis cystoides intestinalis following transperitoneal renal transplantation in a child. Am J Kidney Dis 1984;3:414-9. [Crossref] [PubMed]
- van Son WJ, van der Jagt EJ, van der Woude FJ, et al. Pneumatosis intestinalis in patients after cadaveric kidney transplantation. Possible relationship with an active cytomegalovirus infection. Transplantation 1984;38:506-10. [Crossref] [PubMed]
- Ammons MA, Bauling PC, Weil R 3rd. Pneumatosis cystoides intestinalis with pneumoperitoneum in renal transplant patients on cyclosporine and prednisone. Transplant Proc 1986;18:1868-70. [PubMed]
- Murphy BJ, Weinfeld A. Innocuous pneumatosis intestinalis of the right colon in renal transplant recipients. Report of three cases. Dis Colon Rectum 1987;30:816-9. [Crossref] [PubMed]
- Chelimsky G, Blanchard S, Sivit C, et al. Pneumatosis intestinalis and diarrhea in a child following renal transplantation. Pediatr Transplant 2003;7:236-9. [Crossref] [PubMed]
- Nakamura K, Ohmori Y, Okamoto M, et al. Renal transplant recipient experiencing pneumatosis cystoides intestinalis: a case report. Transplant Proc 2003;35:297-9. [Crossref] [PubMed]
- Pokorny H, Plochl W, Soliman T, et al. Acute colonic pseudo-obstruction (Ogilvie's-syndrome) and pneumatosis intestinalis in a kidney recipient patient. Wien Klin Wochenschr 2003;115:732-5. [Crossref] [PubMed]
- Arora A, Jouhra F. Pneumatosis cystoides intestinalis with pneumoperitoneum in a renal transplant patient. Br J Hosp Med (Lond) 2014;75:407. [Crossref] [PubMed]
- Janssen DA, Kalayoglu M, Sollinger HW. Pneumatosis cystoides intestinalis following lactulose and steroid treatment in a liver transplant patient with an intermittently enlarged scrotum. Transplant Proc 1987;19:2949-52. [PubMed]
- Sachse RE, Burke GW 3rd, Jonas M, et al. Benign pneumatosis intestinalis with subcutaneous emphysema in a liver transplant recipient. Am J Gastroenterol 1990;85:876-9. [PubMed]
- King S, Shuckett B. Sonographic diagnosis of portal venous gas in two pediatric liver transplant patients with benign pneumatosis intestinalis. Case reports and literature review. Pediatr Radiol 1992;22:577-8. [Crossref] [PubMed]
- Burress GC, Ben-Ami T, Whitington PF. Pneumatosis intestinalis in infants after orthotopic liver transplantation. J Pediatr Gastroenterol Nutr 1996;23:577-82. [Crossref] [PubMed]
- Halkic S, Zeini S, Mosimann F, et al. Cystic pneumatosis of the colon after liver transplantation. Minerva Chir 2003;58:385-7. [PubMed]
- Kwon HJ, Kim KW, Song GW, et al. Pneumatosis intestinalis after liver transplantation. Eur J Radiol 2011;80:629-36. [Crossref] [PubMed]
- Abdel-Aziz O, Elaffandi AH, El Shazly M, et al. Pneumatosis intestinalis following pediatric live-related liver transplant: a case report and successful conservative approach. Pediatr Transplant 2014;18:E18-21. [Crossref] [PubMed]
- Fleenor JT, Hoffman TM, Bush DM, et al. Pneumatosis intestinalis after pediatric thoracic organ transplantation. Pediatrics 2002;109:E78-8. [Crossref] [PubMed]
- Mannes GP, de Boer WJ, van der Jagt EJ, et al. Pneumatosis intestinalis and active cytomegaloviral infection after lung transplantation. Groningen Lung Transplant Group. Chest 1994;105:929-30. [Crossref] [PubMed]
- Böhler A, Speich R, Russi EW, et al. Pneumatosis intestinalis and active cytomegaloviral infection after lung transplantation. Chest 1995;107:582-3. [Crossref] [PubMed]
- Schenk P, Madl C, Kramer L, et al. Pneumatosis intestinalis with Clostridium difficile colitis as a cause of acute abdomen after lung transplantation. Dig Dis Sci 1998;43:2455-8. [Crossref] [PubMed]
- Thompson WM, Ho L, Marroquin C. Pneumatosis intestinalis and pneumoperitoneum after bilateral lung transplantation in adults. AJR Am J Roentgenol 2011;196:W273-9. [Crossref] [PubMed]
- Chandola R, Elhenawy A, Lien D, et al. Massive gas under diaphragm after lung transplantation: pneumatosis intestinalis simulating bowel perforation. Ann Thorac Surg 2015;99:687-9. [Crossref] [PubMed]
- Feuerstein JD, White N, Berzin TM. Pneumatosis intestinalis with a focus on hyperbaric oxygen therapy. Mayo Clin Proc 2014;89:697-703. [Crossref] [PubMed]
- Yale CE, Balish E, Wu JP. The bacterial etiology of pneumatosis cystoides intestinalis. Arch Surg 1974;109:89-94. [Crossref] [PubMed]
- Hall RR, Anagnostou A, Kanojia M, et al. Pneumatosis intestinalis associated with graft-versus-host disease of the intestinal tract. Transplant Proc 1984;16:1666-8. [PubMed]
- Tahiri M, Levy J, Alzaid S, et al. An approach to pneumatosis intestinalis: Factors affecting your management. Int J Surg Case Rep 2015;6C:133-7. [Crossref] [PubMed]
- DuBose JJ, Lissauer M, Maung AA, et al. Pneumatosis Intestinalis Predictive Evaluation Study (PIPES): a multicenter epidemiologic study of the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2013;75:15-23. [Crossref] [PubMed]


