Atelectrauma or volutrauma: the dilemma
Introduction
The therapy of acute respiratory distress syndrome (ARDS) remains substantially supportive, aiming to “buy time” for healing while providing adequate gas exchange with the lowest possible damage. Therefore, the choice of tidal volume and positive end-expiratory pressure (PEEP) levels—the most investigated variables within the supportive ventilation—primarily depends on the estimate of the risk associated with their application. The possible damages are collectively referred to as ventilator-induced lung injury (VILI) and their increase or decrease should represent the outcome variable when comparing different ventilatory modes. However, the primary considered outcome is usually mortality, even though any real link between VILI and death has not been identified yet. While a large evidence and consensus support the use of lower tidal volume (6 vs. 12 mL/kg) to decrease VILI (1), the way of setting PEEP is more debated and controversial. Indeed, the extremes of selecting PEEP oscillates between low PEEP values (8–10 cmH2O) as used in daily practice (2), and very high PEEP levels (greater than 20 cmH2O) to “guarantee” a fully open lung (3). A recent trial, the Alveolar Recruitment for ARDS Trial (ART), to surprise of many, showed a significantly higher mortality in patients treated with the open lung strategy, where lung recruitment maneuvers were associated to high PEEP strategy, compared to control patients treated without any recruitment maneuver and with lower PEEP (4). The unexpected results raised discussion, doubts, controversies and uncertainties. In this report, we will refer to what, in our opinion, may be concluded analyzing the results from all the trials performed on the same issue. Our discussion will be focused on the concept of volutrauma, atelectrauma and the mechanical power associated with these strategies.
Lung anatomy
For defining ARDS, bilateral “X-rays infiltrations” must be present (5). Under this definition several lesions differing for nature, distribution and spatial dimension may be present, being X-ray densities the result of mass to volume ratio. A pure atelectasis (severe decreased volume due to the gas loss) has the same density of a consolidation (normal or increased volume where gas has been substituted with liquid/solid material) (6). Densities may be also due to interstitial edema, a primary reason of compression atelectasis in ARDS (7). The characteristics of densities, as distribution and dimension, reflect nature and cause of the disease. Patchy densities sparse in the parenchyma usually reflect pneumonia focuses in which the pulmonary units maintain their volume and are not collapsed. At the opposite, if the cause of the lung disease resides outside the lung (8), the increased lung permeability leads to interstitial edema and, under the increased lung weight, the pulmonary units collapse (7,9). In this case, the lung appears primarily dense in the dependent regions, while the non-dependent are spare.
Opening and closing pressure
Lung opening occurs only in collapsed pulmonary units. In such units, the gas entry requires an opening pressure that is sum of three components (10). The first component is the pressure required to break the inter-molecular water bonds, which cover the inner alveolar surface. This pressure is higher if the surfactant is scarce or absent, as in ARDS, but its order of magnitude usually ranges between 15–20 cmH2O (11). The second component is the pressure necessary to overcome the compressing forces on the pulmonary unit. This pressure cannot be higher than the total lung height in supine position (i.e., 10–15 cmH2O) (7). Finally, the third component is the pressure required to displace the chest wall during lung expansion. This pressure depends on the chest wall elastance and, in normal conditions, should be around 5–10 cmH2O (7). Furthermore, to open a series of neighbor-collapsed units may require further pressure because of their interdependency. Therefore, the resulting opening pressures may range between 45 and 60 cmH2O. It must be noted, however, that the amount of units recruitable between 45 and 60 cmH2O is no more than 1–3% (12). One may ask if there is any clinical meaning in using such pressures with their unavoidable hemodynamic consequences to open an irrelevant fraction of the lung parenchyma. After opening, to keep open a pulmonary unit requires lower pressure. Indeed, while the compressive forces and chest wall elastance equally weight during inspiration and expiration, the pressure necessary to get over the surface tension, for the reasons described before, is no-longer necessary during expiration. Therefore, the pressure to keep fully open the ARDS lung ranges around 25 cmH2O (7).
Recruitment: what it is and how to perform it?
“Recruitment” means the enrollment of an individual unit from a given status to another one. Therefore, to speak the same language and avoid confusion, we should agree on which are the statuses and which are the individual units that we are referring to. In Table 1, we summarize the most relevant status changes described in literature and the methods used to assess them.
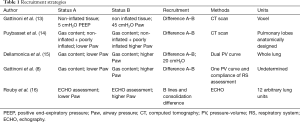
Full table
As shown, under the word “recruitment”, largely different realities have been collected. Not surprisingly, as an example, in the same patient the computed tomography (CT) scan method would give a recruitment of 5% while the single pressure-volume (PV) curve may result in recruitment of 50% (17). The explanation is simple. In one case, we refer to the percentage of lung tissue that actually regains gas measuring it through the difference of non-inflated tissue (voxel). The other one refers to new gas entered in completely degassed pulmonary units plus gas entered in already open units that at higher pressure simply improve their own compliance. Unfortunately, the improvement of compliance is not necessarily due to “recruitment”. This is evident just observing a normal PV curve of a normal lung. At the beginning, more pressure is required to inflate gas in a pulmonary unit simply because the attractive forces of the water molecules (surface tension) are greater when volume is lower. Moving progressively to higher volume, lower change in pressure is required to inflate the same unit just because the surface forces to overcome are decreased. Reaching higher volume, the pressure necessary to inflate the same unit increases again because of the tension of the extracellular fiber network. This phenomenon represents the basis of the sigmoidal shape of the PV curve where the lowest compliance of respiratory system (RS) is located below the lower inflection point (high attractive forces) and above the upper inflection point (high extracellular fiber tension). The concepts of opening pressure and recruitment are often confused while they are distinctly different. “Opening pressure” reflects an intensity property of the system. Indeed, open up a single pulmonary unit compressed by 10 cmH2O requires a pressure greater than 10 cmH2O. The same pressure is required even though the compressed units to open up are not one single unit but are 1,000. In contrast, the efficacy of a recruitment maneuver strictly depends on the number of units transformed from collapsed to aerated. For this reason, “recruitment” reflects a capacitive property of the system. As consequence, for the same “opening pressure” (30 cmH2O), the percentage of effectively recruited tissue may range between 50% and 100% (10). In decades, several recruitment maneuvers have been described, but we think that here is not useful to describe all of them as excellent review of the topic may be found elsewhere (18).
High vs. low PEEP
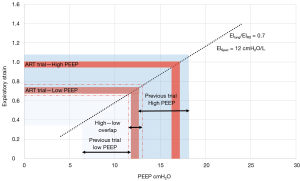
The use of words as “low” and “high” reflects our lack of knowledge and agreement about the PEEP selection. Originally, PEEP was used at levels between 5–10 cmH2O to maintain higher lung volumes and improve gas exchange (19). However, in the 90s PEEP was not considered only as a tool to improve gas exchange but it became a key player in the open lung strategy which aimed to abolish any possible lung damage regardless the gas exchange. We report in Figure 1 the PEEP levels used in the three classical PEEP-trial (21-23) and in the ART trial in relation with expiratory static lung strain. As shown, in the previous trials, the low-PEEP arm corresponds to a strain between 0.3 and 0.7 while, in the ART trial, the low-PEEP arm corresponds to a strain of 0.7. On the other side, in the three-previous trial, the high-PEEP arm is related to a strain between 0.7 and 1.1. While, in the ART trial, the high-PEEP arm corresponds to a strain of 0.95 (we remind that this level corresponds to doubling the end-expiratory lung volume to which a tidal inspiration is over-imposed). Of note, the low-PEEP arm of the ART trial falls in the range of the high-PEEP arm of the previous ones. It clearly appears that terms “low” and “high” are at least inappropriate when discussing PEEP, and may generate confusion.
Atelectrauma and volutrauma
The physiological foundation of “atelectrauma” was theorized by Mead et al. (24). These authors underlined the impact of stress and strain maldistribution in a lung with inhomogeneous pulmonary units. Theoretical analysis suggests that extracellular lung fibers interfacing structures with different elasticity may act as stress risers. Indeed, the stress measured at the airway should be almost duplicated at the interface, as estimated in a CT-scan study (25). Lachman, in his work on inverted ratio ventilation, largely promoted the open lung concept (prevention of atelectasis) to decrease atelectrauma (3), which was documented by Tremblay and coworkers (26) in an experimental setup and by Ranieri and cooperators in a clinical setting (27). Based more on belief than on convincing evidence, atelectrauma was considered as the primary cause of VILI and the open lung strategy the receipt to cure it. Unfortunately, the open lung approach underestimates the hazard of the fully open lung, as PEEP of the order of 20–25 cmH2O is necessary. At such pressures, during inspiration, the lung volumes are close to total lung capacity. Experimentally, such strategy is associated with remarkable lung damages (28). Three previous large randomized studies comparing the possible danger of atelectrauma in patients treated with lower PEEP (range of 7 cmH2O) vs. possible dangers of volutrauma in patients treated with higher PEEP (range of 15 cmH2O) did not show any conclusive evidence (21-23). Therefore, in unselected general ARDS population, the harm/benefit of atelectrauma/volutrauma are equivalent. The ART trial, providing additional data of open lung strategy may contribute to solve the atelectrauma/volutrauma dilemma.
The ART trial
In this multicenter-randomized trial, between November 2011 and April 2017, from 9 countries and 120 intensive care units (ICUs), 1,010 patients were enrolled. The objective of the study was to assess if lung recruitment followed by selection of “best PEEP” decreases 28-day mortality compare to a low PEEP strategy. After the randomization, each patient located in the high PEEP group underwent a first recruitment maneuver using pressure-controlled-ventilation with driving pressure of 15 cmH2O, respiratory rate (RR) of 15 bpm, and incremental PEEP to obtain the airway pressure (Paw) of 40 cmH2O for 1 minute, of 50 cmH2O for 1 minute and of 60 cmH2O for 2 minutes. At the end of the recruitment, to select the PEEP associated with the best compliance each patient underwent a decremented PEEP phase of 15 minutes (3 minutes and 3 cmH2O each step) using volume-control ventilation. However, after three episodes of resuscitated cardiac arrest in the experimental group, the protocol was modified with a reduced recruitment maneuver [pressure control ventilation (PCV), PEEP levels of 25, 30, 35 cmH2O, step of 1 minute, ΔPaw =15, RR =15]. Both groups received volume assist-control ventilation until weaning. In 120 cases (25%), the recruitment maneuver was interrupted because of hypotension, oxygen desaturation or pneumothorax. In 393 patients (78.4%), the recruitment was repeated immediately after the PEEP selection and in 179 patients (37.3%) it was repeated during the first 7 days. Length of ICU/hospital stay, rates of death with refractory hypoxemia and acidosis were not statistically significant between groups. The 28-day mortality was statistically higher in the PEEP group (55.3% vs. 49.3%, P=0.041) as well as all-cause mortality within 6 months (65.3% vs. 59.9% P=0.04). Despite not statistically significant difference in ICU mortality, the highest number of ICU deaths within 7 days was in the experimental arm (31.9% vs. 25.5%, P=0.03).
The ART trial: why did it fail?
The ART trial failed to show benefit in the open lung strategy. Within the possible explanations are: (I) a reduction in driving pressure, in the treatment arm, far lower than expected leading to an under-powered study and to unbalance between reduction in driving pressure and over-distention, during the recruitment maneuver; (II) too high level of PEEP in the control group (12 cmH2O day 1, see Figure 1) which may have prevented atelectrauma; (III) the breath-stacking phenomenon in volume assist-control ventilation. These reasons are all plausible and would be acceptable if the outcome of the experimental and control groups would have been the same. Actually, no one accounts for the survival advantage in the control group treated with lower PEEP. The hazardous way to perform the recruitment maneuver may have had an impact on the dismal outcome of the treatment group. The role of higher PEEP in the worse outcome group cannot be ignored.
The ART trial and the mechanical power
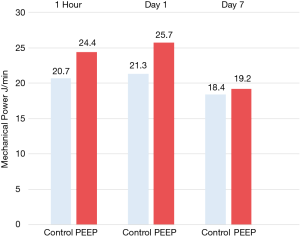
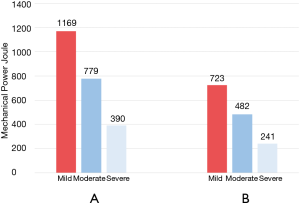
The trial may be also analyzed in the framework of the mechanical power hypothesis (29). As shown in Figure 2, 1 hour after the randomization, mean mechanical power for the PEEP group was 24.4 J/min and mean mechanical power for the control group was 20.7 J/min with a difference of 3.7 J/min between the two groups. At day 1, the mean mechanical power of the PEEP group reached 25.7 J/min while the control group reached 21.3 J/min with a difference of 4.4 J/min. At day 7, both groups had a decreased mean mechanical power (18.4–19.2 J/min) without a difference of 0.8 J/min. Actually, we do not know if a difference of 4–5 J/min for a week of ventilation is enough to justify the major mortality in the PEEP group. However, it is clear that a “protective strategy” targeted to minimize the driving pressure using the best PEEP approach and leaving aside the effective energy delivered to the lung can dramatically result in a conscious or unconscious “harmful strategy” like in the ART trial. Another aspect is represented by the recruitment maneuver placed at least once in PEEP group patients. In Figure 3, we report for mild, moderate and severe ARDS patients, the energy delivered to the lung during the recruitment, before and after the modification of the study protocol. The amount of power is computed assuming a functional residual capacity (FRC) of 1.5 L for mild ARDS, 1.0 L for moderate ARDS and 0.5 L for severe ARDS and assuming that when the Paw is above 40 cmH2O the change in volume reaches the total lung capacity (FRC ×3). Obviously, this computation reflects the order of magnitude of energy delivered to the lung during overall recruitment maneuver. As shown, this energy is around 10 times greater than the energy delivered by the ventilator in a comparable unit of time. We may wonder if 100 J (4 minutes of ventilation in the treatment group) can be potentially harmful, how harmful could be 1,000 J delivered in 4 minutes of recruitment maneuver?
Conclusions
When we compere two different ventilatory modes in ARDS we compare their weight in producing “VILI” although a precise definition of VILI and its link with the mortality are far to be understood. For what we know now, we compare the possible prevention of the two most important triggers of VILI: atelectrauma and volutrauma. The results of available studies, in our opinion, lead to straightforward conclusion. Atelectrauma, which should be greater at PEEP around 7 cmH2O, leads the same outcome of volutrauma, which should be greater at PEEP around 15 cmH2O, as shown by the three randomized large trials on PEEP (21-23). The ART trial just showed that increasing PEEP above 15 cmH2O, particularly if associated with an eccentric recruitment maneuver, is dangerous and increases mortality. In normal practice, the PEEP applied in severe ARDS all around the world, is 8 to 10 cmH2O (2). This should tell us something. Thanks to the ART trial, we may corroborate the suspect that we had before: open lung strategy is an appealing dream, but a dangerous practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Lachmann B. Open up the lung and keep the lung open. Intensive Care Med 1992;18:319-21. [Crossref] [PubMed]
- Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators, Cavalcanti AB, Suzumura ÉA, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017;318:1335-45. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Gattinoni L, Caironi P, Pelosi P, et al. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med 2001;164:1701-11. [Crossref] [PubMed]
- Cressoni M, Chiumello D, Carlesso E, et al. Compressive forces and computed tomography-derived positive end-expiratory pressure in acute respiratory distress syndrome. Anesthesiology 2014;121:572-81. [Crossref] [PubMed]
- Gattinoni L, Pelosi P, Suter PM, et al. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med 1998;158:3-11. [Crossref] [PubMed]
- Pelosi P, D’Andrea L, Vitale G, et al. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am J Respir Crit Care Med 1994;149:8-13. [Crossref] [PubMed]
- Cressoni M, Chiumello D, Algieri I, et al. Opening pressures and atelectrauma in acute respiratory distress syndrome. Intensive Care Med 2017;43:603-11. [Crossref] [PubMed]
- Ghadiali SN, Gaver DP. Biomechanics of liquid-epithelium interactions in pulmonary airways. Respir Physiol Neurobiol 2008;163:232-43. [Crossref] [PubMed]
- Borges JB, Okamoto VN, Matos GF, et al. Reversibility of lung collapse and hypoxemia in early acute respiratory distress syndrome. Am J Respir Crit Care Med 2006;174:268-78. [Crossref] [PubMed]
- Gattinoni L, Pesenti A, Avalli L, et al. Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am Rev Respir Dis 1987;136:730-6. [Crossref] [PubMed]
- Puybasset L, Cluzel P, Chao N, et al. A computed tomography scan assessment of regional lung volume in acute lung injury. The CT Scan ARDS Study Group. Am J Respir Crit Care Med 1998;158:1644-55. [Crossref] [PubMed]
- Dellamonica J, Lerolle N, Sargentini C, et al. PEEP-induced changes in lung volume in acute respiratory distress syndrome. Two methods to estimate alveolar recruitment. Intensive Care Med 2011;37:1595-604. [Crossref] [PubMed]
- Rouby JJ, Arbelot C, Brisson H, et al. Measurement of alveolar recruitment at the bedside: the beginning of a new era in respiratory monitoring? Respir Care 2013;58:539-42. [Crossref] [PubMed]
- Chiumello D, Marino A, Brioni M, et al. Lung Recruitment Assessed by Respiratory Mechanics and Computed Tomography in Patients with Acute Respiratory Distress Syndrome. What Is the Relationship? Am J Respir Crit Care Med 2016;193:1254-63. [Crossref] [PubMed]
- Fan E, Wilcox ME, Brower RG, et al. Recruitment maneuvers for acute lung injury: a systematic review. Am J Respir Crit Care Med 2008;178:1156-63. [Crossref] [PubMed]
- Falke KJ, Pontoppidan H, Kumar A, et al. Ventilation with end-expiratory pressure in acute lung disease. J Clin Invest 1972;51:2315-23. [Crossref] [PubMed]
- Chiumello D, Carlesso E, Cadringher P, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med 2008;178:346-55. [Crossref] [PubMed]
- Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:637-45. [Crossref] [PubMed]
- Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:646-55. [Crossref] [PubMed]
- Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327-36. [Crossref] [PubMed]
- Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 1970;28:596-608. [Crossref] [PubMed]
- Cressoni M, Chiumello D, Chiurazzi C, et al. Lung inhomogeneities, inflation and [18F]2-fluoro-2-deoxy-D-glucose uptake rate in acute respiratory distress syndrome. Eur Respir J 2016;47:233-42. [Crossref] [PubMed]
- Tremblay L, Valenza F, Ribeiro SP, et al. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 1997;99:944-52. [Crossref] [PubMed]
- Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999;282:54-61. [Crossref] [PubMed]
- Protti A, Andreis DT, Milesi M, et al. Lung anatomy, energy load, and ventilator-induced lung injury. Intensive Care Med Exp 2015;3:34. [Crossref] [PubMed]
- Gattinoni L, Tonetti T, Cressoni M, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med 2016;42:1567-75. [Crossref] [PubMed]


