Left ventricular assist device (LVAD) program in Chile: first successful experience in South America
Introduction
Chile has a vast history of cardiac surgery, particularly in the field of heart transplantation (HT). As a matter of fact, the year 2018 marks the 50th anniversary of the first HT surgery in the country, performed on July 28th, 1968 by Dr. Jorge Kaplan, in the region of Valparaíso only 6 months after the world’s first HT by Dr. Christiaan Barnard in South Africa. A few years later, Dr. Kaplan created the department of cardiovascular surgery in our hospital, located in the city of Viña del Mar. This unit is now responsible for the treatment of patients diagnosed with end-stage heart failure (ES-HF) over a population of more than 10 million beneficiaries of the Chilean public health care system.
In over 20 years since the creation of this program more than 100 HT surgeries have been performed, representing the most important figure in the country. The main reason for the reduced number of transplants is the low donation rate, which hardly reaches a rate of 7 per million habitants, of which only 20% become heart donors (1). This limitation implies that many patients die while waiting on the national HT list. This fact motivated our team to search for new treatment alternatives for patients who arrived at our hospital seeking for a solution to their ES-HF.
The new technologies in ventricular assist devices developed during recent years and the better outcomes in survival rates have allowed an important increase in device implantations worldwide (2,3).
We were particularly interested in a third-generation pump, the HeartWare ventricular assist device (HVAD®) (Heart-Ware Inc., USA: HVAD) a small (50 mL) volume, continuous flow-centrifugal pump, with a magnetically and hydrodynamically suspended impeller, with a maximum external diameter of 53 mm and a total weight of 140 grams (4).
There have been reports of promising results with less invasive surgical techniques, allowing a reduction in the number of complications and better preservation of right ventricular (RV) function, usually a very critical factor in patient outcomes, as evidenced by the publications from the Hannover group (5-7).
Our first goal was to obtain funding to install the first two devices and at the same time to present the LVAD project to the sanitary authorities of the country, with the aim of starting a long-term official program. As a result, nine devices have been implanted (8) to date and it is therefore important to review the results. Despite the small number of patients documented in this article, it constitutes the first and most comprehensive review of experience with LVAD implantation in South America.
Methods
Patients
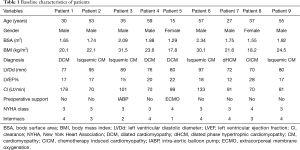
Nine patients (seven males and two females) with a mean age 39±15 years of age, functional class III to IV of the New York Heart Association (NYHA), were chosen for this pilot program. All patients received conventional therapy; eight were on the national HT list including one patient with history of femoral osteosarcoma and carrier of hepatitis C was considered for LVAD as destination therapy. All patients signed the informed consent approved by the Ethics Committee of Gustavo Fricke Hospital in Viña del Mar (Table 1).
Full table
Preoperative status
All patients underwent a thorough evaluation by a multi-disciplinary ES-HF and HT Committee, which decides and defines the indication and enrollment of patients in the national HT list. During LVAD implantation, one patient was classified as Intermacs profile 1, another as Intermacs profile 2, two as Intermacs profile 3 and five as Intermacs profile 4.
The etiology of ES-HF was ischemic cardiomyopathy in four patients, idiopathic cardiomyopathy in three patients, hypertrophic cardiomyopathy in dilated phase in one patient and chemotherapy induced cardiomyopathy in another patient. The mean ejection fraction was 18%. One patient had ECMO support 10 days prior to the intervention; another patient was assisted by balloon counter-pulsation for 72 hours before the surgery.
All patients received an infusion of levosimendan at a dosage of 0.1 µg/kg/min before surgery and the rest of the preoperative preparation was done according to the established local protocol for patients who undergo cardiac surgery.
Surgical technique
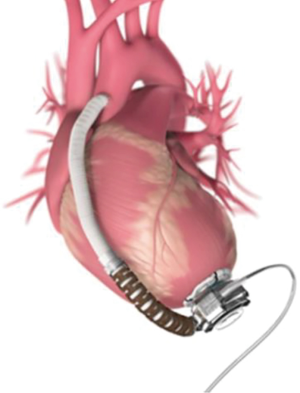
The HVAD® consists of a continuous-flow centrifugal pump, a 21-mm diameter integrated inflow cannula, an outflow Dacron graft, an electrical wire connected to a power source and an external controller that monitors and controls the device. In its interior there is an impeller that suspends and rotates by hydrodynamic and passive magnetic forces, levitating to avoid friction with an adjustable speed between 1,800 and 4,000 rpm, generating flows up to 10 liters per minute. A titanium sewing ring covered in polyester is attached to the apex of the left ventricle (LV) fixing the pump to its position. The externally worn controller—transported by the patient—displays information on speed, flow and energy consumption. It also displays alarms and recommended troubleshooting. The controller is powered by two lithium ion batteries, an AC adaptor, or a DC adaptor for cars.
The preparation for surgery is similar to a conventional cardiac surgery. There are two surgical incisions; the first one is 8-cm long and located under the left side, 5th intercostal space and the second one is an “L” shaped 6-cm long incision at the level of the sternal manubrium. The LV is approached through the left thoracotomy, installing the sewing ring to the anterior side near the apex (9). The outflow graft with the strain relief is attached to the ascending aorta through the second incision. The integrated inflow cannula is installed inside the LV and directed towards the mitral valve, to receive the blood flow that comes from the left atrium (Figure 1). The driveline emerges from the pump and throughout a subcutaneous course in the abdomen; it then exits the patient’s skin at the left flank. It then connects to the controller and two batteries that the patient transports in a specially designed bag. The final installation has been illustrated in Figure 2.
Results
Surgical results
All the implants were performed without major setbacks. In eight patients the pump was implanted through the left anterolateral thoracotomy and one patient required a full sternotomy approach due to her precarious hemodynamic condition prior to surgery (Intermacs profile 1).
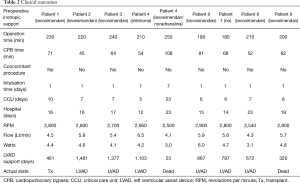
The mean surgery time was 213±26 minutes. The cardiopulmonary bypass time was 67±20 minutes. The initial LVAD flow was about 5 liters per minute, achieved with a speed of 2,700 rpm (Table 2).
Full table
In one patient a supplementary mini-thoracotomy was performed to expose the left atrium and explore a suspected thrombus formation that finally corresponded to a false image in the transesophageal echocardiography.
Post-operative follow- up
One patient (Intermacs profile 1) died 23 days after the implantation surgery due to multiple organ failure. Another patient died after 11 months due to stroke complications; two patients required surgical re-exploration due to bleeding complications in the immediate postoperative period.
Eight patients were extubated within the first 48 hours after surgery. All patients received postoperative adrenalin infusion, adjusting dosage until achieving acceptable hemodynamic conditions and adequate performance of the right ventricle. Long-term survival rate at 6, 12 and 18 months follow-up in this series was 89%, 78% and 78% respectively, similar to the success rate reported in the ReVOLVE study (10)
The most frequent complication observed during follow-up was gastrointestinal bleeding, followed by driveline infections and stroke. One case of device thrombosis was successfully resolved by a single dose of alteplase.
One patient developed outflow graft stenosis that was successfully resolved through endovascular implantation of a 10-mm stent. There were no patients who required extraordinary mechanical support or HVAD® exchange. At the time of this report, five patients were classified as NYHA class I and one with NYHA II (Table 3).
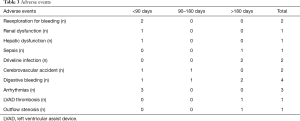
Full table
Discussion
Mechanical circulatory support for patients with advanced heart failure was initiated in the 1960s, however there was no significant progress during the following 4 decades (11). With the incorporation of new LVADs, the number of implants has increased in patients waiting for heart transplantation and as a destination therapy (2,12). The development of LVAD Heartmate II® in the year 2003, increased the number of implants in developed countries reaching more than 20,000 patients with LVADs by June 2017, according the last Intermacs registry report (13).
All patients reported in this study had a waiting time for heart transplantation of over 6 months, and most of them were classified as Intermacs profile 3, with high risk of hospitalization and death. This background prompted the team to create this program with the collaboration of experts from the Hannover Medical School, who guided the first implants and advised on the use of minimally invasive surgical technique developed by Schmitto et al. (8,9,14,15), that offers better survival rate, less morbidity and lower hospitalization costs (5) and a reduction of the complexity in redo operations for heart transplantation (16).
The previously described technique allows maintaining an intact pericardium, preventing dilatation and dysfunction of the RV during the weaning of cardiopulmonary bypass, reducing the need for drugs and/or mechanical support; thus, simplifying the postoperative management and reducing the postoperative hospital stay. One of the central goals of preoperative care is the improvement of contractibility of both ventricles and the reduction in pulmonary hypertension. That objective was achieved by the infusion of levosimendan, whose beneficial effects persist for several days after infusion (17). During surgery, it was critical to accurately monitor and manage preload and volemia, including invasive systemic and pulmonary blood pressure monitoring and transesophageal echocardiography. Most cases received dobutamine and adrenalin infusion to improve contractility and inhaled nitric oxide and iloprost (Ventavis®) to diminish the RV afterload, allowing a better RV function and a more rapid adaptation to the increased LV output generated by the LVAD (18). The weaning from cardiopulmonary bypass was done carefully, slowly restoring the cardiac output generated by the device from the left side of the heart. During the first 24 hours after surgery constant monitoring of signs of RV claudication was necessary to calibrate the device output to achieve an adequate clinical response.
Postoperative hemorrhage is another serious complication, therefore it is considered necessary to use blood by-products, such as prothrombin complex concentrate, fibrinogen, antifibrinolytics and surgical hemostats (19). In this series, patients received prothrombin extracts (Octaplex®), to avoid blood transfusions and reduce the risk of sensitization facing transplantation (20). Another potential risk is infection, and prevention includes preoperative skin wash with antiseptics, oral chlorhexidine, nasal antibiotics and broad spectrum intravenous antibiotics (21).
Most patients evolved without major immediate post-operative events, and the median postoperative length of stay was 17 days, assuring optimum levels of anticoagulation and suitable education to patients and caregivers on the use of the equipment.
The mean follow-up was 846±479 days (range, 23–1,481 days), and has not been devoid of complications, registering a total of 20 hospitalizations.
The patient who died 23 days after surgery was the most severely ill, classified as Intermacs 1, corresponding to the group of patients reported with the highest probability of death because of poor hemodynamic conditions on other organs, that finally lead to multiorgan failure (22).
As described in literature, this group also presented different complications during follow-up, many of them requiring hospitalization, making it necessary to have a close follow-up of these patients. It is probable that future progress in device design and medical treatment will decrease the postoperative complication rate (23).
Conclusions
LVAD implantation as a “bridge to transplantation” is a strategy that has generated considerable interest in the developed world, as a product of the organ donation shortage along with improved cost-effectiveness of new devices (24,25).
This report, even if includes a small number of patients, is the largest in South America and reveals the advantages and difficulties of this important procedure, that has attained great progress but is not free of complications.
The patients described in this study represent the most important experience in minimally invasive LVAD implantation surgery as a bridge to transplantation with the longest follow-up in Chile and South America. The positive outcome of this program is a result of the cumulative experience in ES-HF management, support from successful academic exchange with the Hannover Medical School, and work done for over 20 years in short-term circulatory assistance in advanced heart failure patients (26).
Acknowledgements
The authors are particularly grateful to Professor Axel Haverich, Head of the Cardiac, Thoracic, Transplantation and Vascular Surgery Department, Hannover Medical, Professor Jan Schmitto and Dr. Sebastian Rojas, from the same institution, who gave us their full collaboration, expertise and support to achieve this successful experience.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of Hospital Dr. Gustavo Fricke (No. 16199).
References
- Aránguiz-Santander E, Merello L, Pedemonte O, et al. Heart transplantation in Chile: preliminary report from the Gustavo Fricke hospital in Vina del Mar. Transplant Proc 2007;39:619-21. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015;34:1495-504. [Crossref] [PubMed]
- Schmitto JD, Zimpfer D, Fiane AE, et al. Long-term support of patients receiving a left ventricular assist device for advanced heart failure: a follow-up analysis of the Registry to Evaluate the HeartWare Left Ventricular Assist System. Eur J Cardiothorac Surg 2016;50:834-8. [Crossref] [PubMed]
- Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med 2017;376:451-60. [Crossref] [PubMed]
- Rojas SV, Avsar M, Hanke JS, et al. Minimally-Invasive Implantation of Left Ventricular Assist Devices Improves the Operative Outcome in Adult Patients with Severe Heart Failure. Thorac Cardiovasc Surg 2013;61:OP52. [Crossref]
- Maltais S, Anwer LA, Tchantchaleishvili V, et al. Left Lateral Thoracotomy for Centrifugal Continuous-Flow Left Ventricular Assist Device Placement: An Analysis from the Mechanical Circulatory Support Research Network. ASAIO J 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Rojas SV, Hanke JS, Avsar M, et al. Left Ventricular Assist Device Therapy for Destination Therapy: Is Less Invasive Surgery a Safe Alternative? Rev Esp Cardiol (Engl Ed) 2018;71:13-7. [Crossref] [PubMed]
- Pedemonte O, Vera A, Schmitto JD, et al. Left ventricular assistant devices for end-stage heart failure: report of two cases. Rev Med Chil 2014;142:914-8. [Crossref]
- Schmitto JD, Molitoris U, Haverich A, et al. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg 2012;143:511-3. [Crossref] [PubMed]
- Strueber M, Larbalestier R, Jansz P, et al. Results of the post-market Registry to Evaluate the HeartWare Left Ventricular Assist System (ReVOLVE). J Heart Lung Transplant 2014;33:486-91. [Crossref] [PubMed]
- De Bakey ME, Liotta D, Hall W. Prospects for implications of the artificial heart and assistant devices. J Rehab 1966;32:106-7. [PubMed]
- Kherani AR, Maybaum S, Oz MC. Ventricular assist devices as a bridge to transplant or recovery. Cardiology 2004;101:93-103. [Crossref] [PubMed]
- Intermacs. Quarterly Statistical Report 2017 Q2. Available online: http://www.uab.edu/medicine/intermacs/images/Federal_Quarterly_Report/Federal_Partners_Report_2017_Q2.pdf
- Hanke JS, Rojas SV, Cvitkovic T, et al. First results of HeartWare left ventricular assist device implantation with tunneling of the outflow graft through the transverse sinus. Interact Cardiovasc Thorac Surg 2017;25:503-8. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Avsar M, et al. HeartWare left ventricular assist device for the treatment of advanced heart failure. Future Cardiol 2016;12:17-26. [Crossref] [PubMed]
- Schmitto JD, Rojas SV, Hanke JS, et al. Minimally invasive left ventricular assist device explantation after cardiac recovery: surgical technical considerations. Artif Organs 2014;38:507-10. [Crossref] [PubMed]
- Follath F, Cleland JG, Just H, et al. Steering committee and Investigators of the Levosimendan Infusion versus Dobutamine (LIDO) Study. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low output heart failure (the LIDO study): a randomized double-blind trial. Lancet 2002;360:196-202. [Crossref] [PubMed]
- Lahm T, McCaslin CA, Wozniak TC, et al. Medical and surgical treatment of acute right ventricular failure. J Am Coll Cardiol 2010;56:1435-46. [Crossref] [PubMed]
- Riha H, Fassl J, Patel P, et al. Major themes for 2010 in cardiothoracic and vascular anesthesia. HSR Proc Intensive Care Cardiovasc Anesth 2011;3:33-43. [PubMed]
- Joyce DL, Southard RE, Torre-Amione G, et al. Impact of left ventricular assist device (LVAD)-mediated humoral sensitization on post-transplant outcomes. J Heart Lung Transplant 2005;24:2054-9. [Crossref] [PubMed]
- Acharya MN, Som R, Tsui S. What is the optimum antibiotic prophylaxis in patients undergoing implantation of a left ventricular assist device? Interact Cardiovasc Thorac Surg 2012;14:209-14. [Crossref] [PubMed]
- Abu Saleh WK, Jabbari OA, Guha A, et al. Treatment strategies for patients with an INTERMACS I profile. Methodist Debakey Cardiovasc J 2015;11:4-8. [Crossref] [PubMed]
- Pinney SP, Anyanwu AC, Lala A, et al. Left Ventricular Assist Devices for Lifelong Support. J Am Coll Cardiol 2017;69:2845-61. [Crossref] [PubMed]
- Slaughter MS, Bostic R, Tong K, et al. Temporal changes in hospital costs for left ventricular assist device implantation. J Card Surg 2011;26:535-41. [Crossref] [PubMed]
- Alba AC, Alba LF, Delgado DH, et al. Cost-Effectiveness of Ventricular Assist Device Therapy as a Bridge to Transplant in Comparison to Non-Bridged Cardiac Recipients. Circulation 2013;127:2424-35. [Crossref] [PubMed]
- Pedemonte V O, Aránguiz Santander E, Torres H H, et al. Right ventricular assistance with a centrifugal pump. Report of two cases. Rev Med Chil 2008;136:359-66. [Crossref]