Comparison of short-term outcomes for connective tissue disease-related interstitial lung disease and idiopathic pulmonary fibrosis after lung transplantation
Introduction
Connective tissue disease-related interstitial lung disease (CTD-ILD) has been described in association with many CTDs, including systemic sclerosis (SSc), rheumatoid arthritis (RA), dermatomyositis (DM) or polymyositis (PM), systemic lupus erythematosus (SLE), Sjögren’s syndrome (SjS), and mixed CTD (MCTD) (1-6). Pulmonary involvement can occur in many ways in CTD, but ILD and pulmonary hypertension are the most common manifestations. Thus, respiratory failure is the most common outcome of ILD and is a major cause of morbidity and mortality in CTD-ILD (3-6).
CTD-ILD is difficult to treat due to the nature of the underlying disease and progression to end-stage lung disease that can occur despite active immunosuppressive therapy (1,7,8). Lung transplantation is a potentially life-saving treatment for patients with CTD who have progressed to end-stage pulmonary disease due to ILD (1,7,9-11).
However, despite the poor outcomes for CTD-ILD patients, lung transplantation is performed relatively infrequently in this population. According to data from the International Society for Heart and Lung Transplantation (ISHLT) registry, from January 1995 to June 2012, CTD-associated lung disease accounted for only 1.3% of the 37,581 cases undergoing lung transplantation worldwide (12,13). Many transplantation centres do not offer lung transplantation to patients with CTD because of concerns about the nature of systemic autoimmune diseases and extra-pulmonary involvement that might affect short- and long-term survival (14,15).
Survival, outcomes, and management of these patients after transplantation have been debated. Moreover, there have been few studies of CTD-ILD patients undergoing lung transplantation in Asia (13,16-18).
The primary objective of this study was to compare the post-lung transplant survival of patients with CTD-ILD and those with idiopathic pulmonary fibrosis (IPF), which is a well-established indication for lung transplantation (12). Secondary outcome measures included: (I) the incidence of primary graft dysfunction (PGD) in patients with CTD compared to those with IPF and (II) the pulmonary and extra-pulmonary recurrence of CTD after lung transplantation. This study is important, as it is the first reported comparison of patients with CTD-ILD and IPF undergoing lung transplantation in Asia.
Methods
Study design and population
We performed a retrospective cohort study of adults who underwent lung transplantation at Severance Hospital of Yonsei University between October 2012 and October 2016. We evaluated data from 128 lung transplant recipients who were followed through October 2017.
The diagnoses that were included as CTD-associated lung disorders were SSc, RA, DM, PM, SLE, SjS, and undifferentiated CTD (UCTD). DM and PM were combined into a DM/PM group.
In patients with CTD-ILD, guidelines similar to those of well-known interstitial pneumonia were used as the criteria for selecting lung transplant recipients. Patients with pulmonary hypertension with right ventricular failure worsening were also considered and this was seen in patients with CTD-ILD who differed from IPF patients. In addition, patients with CTD-ILD were associated with extra-pulmonary disease such as renal disease or myositis. Lung transplantation was performed when the disease was controlled by immunosuppressant medication. However, patients with CTD-ILD who were not controlled by medications had frequent underlying CTD flare ups during immunosuppressive therapy and were excluded. Excessive systemic inflammation such as vascular insufficiency and renal failure was also excluded.
In this study, patients requiring mechanical ventilation or extracorporeal membrane oxygenation (ECMO) prior to transplantation were excluded in order to ensure an accurate comparison of patients. All patients who required mechanical ventilation or ECMO due to respiratory failure prior to transplantation were only IPF patients, who were considered to have an effect on outcome comparisons between the two groups and excluded patients who underwent mechanical ventilation or ECMO. We also excluded patients who received combined organ transplants, such as lung-liver transplantation.
Medical records were reviewed for pre-transplant demographics, clinical data, immunological characteristics, acute rejection, complications, and survival. Pulmonary function test, 6-minute walk test, Echocardiogram, right heart catheterization, and high-resolution computed tomography (HRCT) were performed within 1 year before transplantation. Honeycomb scoring was confirmed by reviewing the HRCT of two pulmonologists and dividing the lung into six sections (upper, middle, and lower zones of each lung). Honeycomb score changes determined by visually estimating the percentage from 0% to 100% (at 5% intervals) and averaging them. The assessments of the two pulmonologists were averaged.
Clinical setting
The post-transplant immunosuppressive regimens were based on the protocols of the Yonsei University lung transplantation team. All patients were initially treated with a triple drug regimen consisting of a calcineurin inhibitor, an antimetabolite or purine synthesis inhibitor, and corticosteroids.
Clinical outcomes
Post-transplant data were collected from all patients, including cumulative survival (at 30 days, 6 months, and 1 year), follow-up duration, and incidences of PGD and CTD flare.
PGD was assessed during the first 72 hours after lung transplantation. The severity of PGD was graded based on the ratio of arterial oxygen pressure to inspired oxygen concentration (PaO2/FiO2) and the presence of infiltration on chest radiography according to the ISHLT criteria (19).
CTD flare was defined as a new or progressive condition that could be consistent with the underlying CTD. For all CTDs, symptoms of arthritis, arthralgia, myositis, muscle weakness, vasculitis, fever, thrombocytopenia, skin rashes, dry mouth and eyes, or other organ involvement consistent with the CTD were considered as extra-pulmonary flares. Diagnosis of CTD flare was based on the patient’s clinical history, assessment, and response to treatment. The CTD was considered more likely than another disease to be the cause of a flare.
Statistical analysis
Continuous variables were described using means and standard deviations, and categorical variables were described using counts and percentages. The pre-transplant and post-transplant characteristics of the transplant patients with CTD-related lung disease were compared to those of transplant patients with IPF using Fisher’s exact test and the Mann-Whitney U test for categorical and continuous variables, respectively. Cumulative survival was calculated from the time of transplantation until death, transfer, or the last follow-up appointment. Kaplan-Meier survival curves and log-rank tests were used to compare the survival of the two groups. To confirm an accurate survival rate comparison between the two groups, direct matching was performed using the propensity score matching method. Hazard ratios are expressed as relative risks (RRs) with 95% confidence intervals (95% CIs). Statistical analyses were performed using SPSS version 20 (SPSS, Inc., Chicago, IL, USA). A P value <0.05 was considered statistically significant.
Ethics
The study was approved by the Institutional Review Board (IRB) of Severance Hospital of Yonsei University, with a waiver of individual consent (IRB No. 4-2013-0770).
Results
A total of 128 lung transplantations were performed at Severance Hospital during the study period. However, 44 patients did not have a diagnosis of CTD-ILD or IPF, 21 patients required ECMO and/or mechanical ventilation at the time of lung transplantations, and one patient underwent lung-liver transplantation. Therefore, 62 patients were ultimately included in the study. Fifteen transplantations were performed for patients with CTD-ILD (11.7%), and 47 were performed for those with IPF (36.7%) (Figure 1).
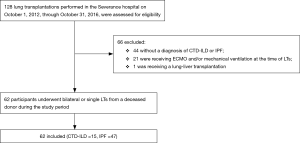
The subtype of CTD-ILD was DM/PM in 5 patients (33.3%), RA in 4 (26.7%), SSc in 3 (20.0%), and SLE, SjS, and UCTD in 1 (6.7%) each.
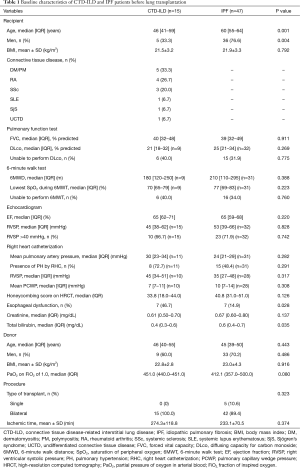
The baseline characteristics and clinical variables of the two groups were compared (Table 1). Compared with patients with IPF, those with CTD-ILD were younger (46 vs. 60 years, P=0.001) and less likely to be male (33.3% vs. 76.6%, P=0.004). The mean pulmonary artery pressure was higher (30 vs. 24 mmHg, P=0.282) and the proportion of patients with pulmonary hypertension was higher in patients with CTD-ILD than in those with IPF (72.7% vs. 48.4%, P=0.291). Honeycombing score on HRCT was high in IPF patients (P=0.126). When the pulmonary function was compared, there was no significant difference between the two groups in forced vital capacity (FVC) (40% vs. 39%, P=0.911) and diffusing capacity (DLco) (21% vs. 25%, P=0.269). Patients with CTD-ILD showed a decrease in a 6-minute walk distance (180 vs. 210 m, P=0.388).
Full table
Endogastroduodenoscopy (EGD), chest computed tomography (CT) and oesophageal manometry were used to confirm oesophageal dysfunction. Clinically oesophageal dysfunction was significantly more frequent in CTD-ILD patients (46.7% vs. 14.9%, P=0.028), and all three patients who showed oesophageal dilatation on CT were CTD-ILD patients. Pre-transplantation laboratory data were within normal limits.
With respect to the donor characteristics, age, sex, and body mass index were similar between both groups. However, PaO2 on FiO2 (P/F ratio) of 1.0 in CTD-ILD group was higher than in the IPF group (P=0.080). All patients with CTD-ILD underwent bilateral lung transplantation, and 10% of IPF patients underwent single lung transplantation, but this difference was not significant (P=0.323) (Table 1).
In 9 of 15 patients with CTD-ILD, pathological findings obtained by transplantation showed suspicious lung involvement of collagen vascular disease by observing lymphoid follicles with usual interstitial pneumonia (UIP) or fibrosing non-specific interstitial pneumonia (NSIP). All patients with IPF were identified with UIP on lung tissue after transplantation, and two patients were found to have diffuse alveolar damage and organizing pneumonia.
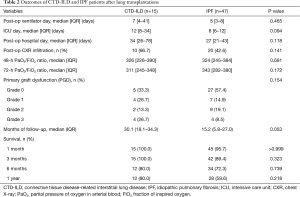
In terms of the immediate outcomes after lung transplantation, patients with CTD-ILD had a longer duration of days in the intensive care unit postoperatively (12 vs. 8, P=0.094) and had more requirements for mechanical ventilators (7 vs. 5, P=0.465), but not significant differences. The presence of infiltrations on chest radiography and the P/F ratio were also similar (Table 2).
Full table
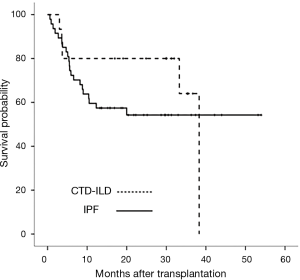
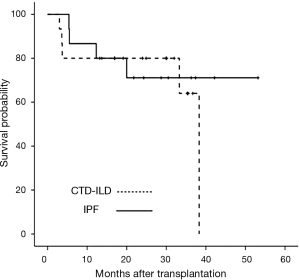
Survival was determined as of October 31, 2017. The median follow-up durations for CTD-ILD and IPF patients were 30.1 and 15.2 months, respectively (Figure 2). During the first 3 months, five patients with IPF (10.6%) died but no patients with CTD-ILD died. The 1-year cumulative survival rate after transplantation was 80.0% in CTD-ILD patients and 59.6% in IPF patients (P=0.218) (Figure 2). Three of the CTD-ILD patients died after 3 months of lung transplantation. One patient underwent bilateral lung transplantation for RA-associated ILD (RA-ILD) and then right lung pneumonectomy due to persistent bronchopleural fistula, but eventually died of bacterial pneumonia with sepsis. Other patient, who also had RA-ILD, died of heart failure due to myopericarditis after lung transplantation. The last patient died of sepsis with gastrointestinal bleeding.
The occurrence of PGD was similar between the two groups. Grade 3 PGD was more common in CTD-ILD patients, but was not statistically significant (26.7% vs. 8.5%, P=0.154).
Because differences in age and sex were evident between the two groups, direct matching through propensity score matching was performed for a more accurate survival comparison. CTD-ILD (n=15) and age- and sex-matched IPF (n=15) patients were directly compared. The median survival durations of CTD-ILD and IPF patients were 29.9 and 21.8 months, respectively. There were no differences in the cumulative survival rates of patients with CTD-ILD and of those IPF over the subsequent 60 months after transplantation (log-rank P=0.613) (Figure 3).
Two (18.2%) patients developed a possible CTD flare during the follow-up period. One patient was diagnosed with PM before transplantation and experienced subacute episodes with upper- and lower-extremity weakness and CO2 retention. The patient received steroids, mycophenolate mofetil as an immunosuppressive agent prior to transplantation, and was taking standard triple immunosuppressive drugs after transplantation. Nerve conduction velocity (NCV) and electromyography (EMG) were performed to distinguish the causes. The NCV results revealed sensorimotor polyneuropathy, and EMG showed generalized myopathy. We increased the dose of steroids, which was effective. Symptoms improved in 2 weeks. The second patient with a possible flare had SLE and showed symptoms of dyspnoea, fever, leukopenia, thrombocytopenia, and hypocomplementemia. Before transplantation, the patient received methylprednisolone and hydroxychloroquine. After transplantation, the patient received a standard post-transplantation triple drug regimen and hydroxychloroquine. The patient also received a high-dose steroid (1 mg/kg) for 2 weeks for the possible flare, and symptoms resolved.
Discussion
Since CTD-ILD is a systemic disease, outcomes, survival, and the possibility of disease deterioration after transplantation have been unclear for affected patients. Thus relatively fewer lung transplantations have been performed for these patients than for patients with other diseases (13).
Many studies have indicated similar survival rates for these patient groups (13,14,16,20-26). Among the studies comparing the survival rates between patients with CTD-ILD and those with other diseases who underwent lung transplantation, Takagishi et al. reported survival outcomes for CTD patients and patients with chronic obstructive pulmonary disease (COPD) and IPF after lung transplantation. The cumulative survival of patients with CTD-ILD was lower than that for patients with COPD at 30 days, 6 months, 1, 2, 3, and 5 years. When compared to the outcomes for IPF patients, cumulative survival was similar at 30 days, 6 months, 2, 3, and 5 years. A significant difference in survival was only noted at 1 year and later (72.7% vs. 77.7%, P=0.049). The authors speculated that CTD-ILD and IPF patients may have been affected by the use of immunosuppressants, including steroids, prior to transplantation, unlike COPD patients who did not receive drugs (18). Similarly, there was no difference in the cumulative survival rate between patients with CTD-ILD and IPF in this study.
Because SSc accounts for most CTD-ILD patients, there have been many studies on SSc patients as a subgroup of CTD-ILD (14,20-26). Saggar et al. retrospectively reported post-lung transplantation outcomes for SSc compared to those for IPF. Fourteen patients with SSc and 38 with IPF underwent lung transplantation. The 1-year all-cause mortality did not differ between SSc (6.6%) and IPF (13.1%) patients (24). A study by Schachna et al. compared outcomes for SSc patients and IPF patients who underwent lung transplantation. Cumulative survival at 6 months after transplantation was 69% in the SSc group and 80% in the IPF group. Over the next 18 months, there was convergence in the survival rates such that the cumulative survival at 2 years was similar (61% and 64%, respectively) (25).
A contradictory result has also been reported (8,10,27). Bernstein et al. performed a retrospective cohort study of 229 adults with SSc, 201 with pulmonary artery hypertension (PAH), and 3,333 with ILD who underwent lung transplantation in the US. SSc patients showed a multivariable-adjusted 48% relative increase in 1-year mortality rate compared to non-SSc-related ILD patients [hazard ratio (HR), 1.48; 95% CI, 1.01–2.17). However, there was no difference in the 1-year mortality risk between patients with SSc and those with non-SSc-related PAH (HR, 0.85; 95% CI, 0.50–1.44) (27).
Because SSc patients, who comprise a large proportion of CTD patients (12), were reported to have higher mortality rates after transplantation than ILD patients, fewer pulmonary transplantations have been performed for CTD-ILD patients due to uncertainty about the results after transplantation (13). Oesophageal dysmotility and gastroesophageal reflux disease, which are common in SSc patients (28), might increase the risk for infection and graft dysfunction (13,29-33). Therefore, CTD-ILD is divided into subtypes, and comparative studies with other diseases have been conducted. In this study, one of four SSc patients showed typical oesophageal dilatation on CT, but oesophageal dysfunction was not observed on manometry, and lung transplantation was conducted. After transplantation, there was no suspicion of bronchiolitis obliterans syndrome (BOS). Two patients were suspected of having oesophageal dilatation on CT, but could not undergo endogastroduodenoscopy due to their conditions. They survived without complications after transplantation. In patients with CTD-ILD, clinically suspected oesophageal dysfunction was present, but no patient showed complications due to esophageal dysfunction during the follow-up period after lung transplantation.
Recent studies have reported the results of transplantation for non-SSc-ILD patients (14,34). Courtwright et al. conducted a retrospective study of outcomes after transplantation in patients with non-SSc-ILD and IPF. Non-SSc-ILD patients did not have worse adjusted survival rates (HR, 1.14; 95% CI, 0.92–1.42) (34).
There are also comparative studies on other subtypes of CTD-ILD. Yazdani et al. analysed post-transplant survival in RA-ILD, IPF, and SSc-ILD patients. Cumulative survival at 1-year post-transplantation for the RA-ILD, IPF, and SSc-ILD groups were 67%, 69%, and 82%, respectively (log-rank P=0.7). There was no significant difference among groups in age- and sex-adjusted analyses (P=0.900) (35). Ameye et al. reported their experience with patients with ILD associated with idiopathic inflammatory myopathies (IIM), such as DM or PM, who underwent transplantation. The 1-year survival rate was 100%, and the 2- and 5-year survival rates were 75% compared with 86%, 67%, and 58%, respectively, for IPF patients undergoing lung transplantation. Respective rates were 86%, 63%, and 57% for patients undergoing transplantation for non-IPF- and non-IIM-related ILD (36). In this study, the median survival rate of 5 DM/PM patients (33.3%) was 32.6 months, and one of them died of pneumonia at 38 months postoperatively. These results together suggest that lung transplantation can be a valid option for RA-ILD and ILD-related DM/PM.
In our study, the outcomes after lung transplantation for CTD-ILD patients were similar to those for IPF patients in concurrent cohorts of transplant recipients at the same medical centre, even when adjusting for important factors such as age and sex. There were no differences in the cumulative survival or incidence of PGD between the two groups. After transplantation, two patients experienced possible flare of CTD, even though CTD is highly unlikely to flare.
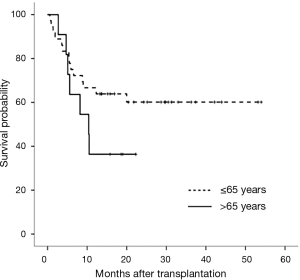
This study had some limitations. First, a small number of patients with CTD-ILD or IPF were enrolled, and the analysis was conducted in one institution. Although this study accounts for a large distribution of lung transplantations performed in Asia, it is still considered as a relatively small-scale study worldwide. Additional multi-centre analyses are needed. Second, the 1-year cumulative survival rate of IPF patients was relatively low in this study. Survival risk factors for IPF patients were identified as age. The survival rates were different between the patients under 65 and over 65 years of age (log-rank P=0.162) (Figure 4). These results suggest that the survival rates of older IPF patients and the overall survival rates of IPF patients are low.
Third, pre-transplant right heart catheterization, which can accurately identify pulmonary hypertension in CTD-ILD patients, was not performed in all patients. Moreover, in a literature review, the survival rate for SSc varied according to the study (67.6–93.4% survival at 1-year post-transplantation) (14,24), but we did not analyse the subtypes of CTD in this study. For example, it would have been more accurate to identify patients with pulmonary hypertension who did not have ILD. Because of the multifactorial characteristics of the CTD disease group, it would be better to analyse the subtype of CTD disease. In addition, we reported only short-term (at 30 days, 6 months, and 1 year) results. For a more accurate analysis, including long-term complications, such as chronic lung allograft dysfunction, long-term survival data are needed. Finally, our study did not identify the factors associated with mortality following lung transplantation in each group because only three patients died. CTD-associated morbidity that can cause death should have been considered in the analysis.
However, the strength of this study is that it is the first to compare CTD-ILD lung transplantation recipients to lung transplantation recipients with other diseases in Asia. We confirmed the occurrence of CTD flare, but it was not a serious complication.
As the use of lung transplantation increases worldwide, lung transplantation in patients with CTD is also expected to increase in the future. In Asia, where there are still insufficient data, additional data collection, comparison, and analysis through prospective and multi-centre studies on the subgroups of CTD-ILD are considered necessary.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board (IRB) of Severance Hospital of Yonsei University (IRB No. 4-2013-0770).
References
- Castelino FV, Varga J. Interstitial lung disease in connective tissue diseases: evolving concepts of pathogenesis and management. Arthritis Res Ther 2010;12:213. [Crossref] [PubMed]
- Hant FN, Herpel LB, Silver RM. Pulmonary manifestations of scleroderma and mixed connective tissue disease. Clin Chest Med 2010;31:433-49. [Crossref] [PubMed]
- Antin-Ozerkis D, Evans J, Rubinowitz A, et al. Pulmonary manifestations of rheumatoid arthritis. Clin Chest Med 2010;31:451-78. [Crossref] [PubMed]
- Kalluri M, Oddis CV. Pulmonary manifestations of the idiopathic inflammatory myopathies. Clin Chest Med 2010;31:501-12. [Crossref] [PubMed]
- Kamen DL, Strange C. Pulmonary manifestations of systemic lupus erythematosus. Clin Chest Med 2010;31:479-88. [Crossref] [PubMed]
- Kokosi M, Riemer EC, Highland KB. Pulmonary involvement in Sjogren syndrome. Clin Chest Med 2010;31:489-500. [Crossref] [PubMed]
- de Lauretis A, Veeraraghavan S, Renzoni E. Review series: Aspects of interstitial lung disease: connective tissue disease-associated interstitial lung disease: how does it differ from IPF? How should the clinical approach differ? Chron Respir Dis 2011;8:53-82. [Crossref] [PubMed]
- Kocheril SV, Appleton BE, Somers EC, et al. Comparison of disease progression and mortality of connective tissue disease-related interstitial lung disease and idiopathic interstitial pneumonia. Arthritis Rheum 2005;53:549-57. [Crossref] [PubMed]
- Mathai SC, Hummers LK, Champion HC, et al. Survival in pulmonary hypertension associated with the scleroderma spectrum of diseases: impact of interstitial lung disease. Arthritis Rheum 2009;60:569-77. [Crossref] [PubMed]
- Park JH, Kim DS, Park IN, et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med 2007;175:705-11. [Crossref] [PubMed]
- Ryu JH, Bongartz T, Matteson EL. Interstitial lung disease in connective tissue diseases: what are the important questions? Arthritis Rheum 2005;53:488-90. [Crossref] [PubMed]
- Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:965-78. [Crossref] [PubMed]
- Lee JC, Ahya VN. Lung transplantation in autoimmune diseases. Clin Chest Med 2010;31:589-603. [Crossref] [PubMed]
- Massad MG, Powell CR, Kpodonu J, et al. Outcomes of lung transplantation in patients with scleroderma. World J Surg 2005;29:1510-5. [Crossref] [PubMed]
- Anderson LA, Gadalla S, Morton LM, et al. Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. Int J Cancer 2009;125:398-405. [Crossref] [PubMed]
- Khan IY, Singer LG, de Perrot M, et al. Survival after lung transplantation in systemic sclerosis. A systematic review. Respir Med 2013;107:2081-7. [Crossref] [PubMed]
- Yeatman M, McNeil K, Smith JA, et al. Lung Transplantation in patients with systemic diseases: an eleven-year experience at Papworth Hospital. J Heart Lung Transplant 1996;15:144-9. [PubMed]
- Takagishi T, Ostrowski R, Alex C, et al. Survival and extrapulmonary course of connective tissue disease after lung transplantation. J Clin Rheumatol 2012;18:283-9. [Crossref] [PubMed]
- Christie J, Keshavjee S, Orens J, et al. Potential refinements of the International Society for Heart and Lung Transplantation primary graft dysfunction grading system. J Heart Lung Transplant 2008;27:138. [Crossref] [PubMed]
- Kubo M, Vensak J, Dauber J, et al. Lung transplantation in patients with scleroderma. J Heart Lung Transplant 2001;20:174-5. [Crossref] [PubMed]
- Levine SM, Anzueto A, Peters JI, et al. Single lung transplantation in patients with systemic disease. Chest 1994;105:837-41. [Crossref] [PubMed]
- Pigula FA, Griffith BP, Zenati MA, et al. Lung transplantation for respiratory failure resulting from systemic disease. Ann Thorac Surg 1997;64:1630-4. [Crossref] [PubMed]
- Rosas V, Conte JV, Yang SC, et al. Lung transplantation and systemic sclerosis. Ann Transplant 2000;5:38-43. [PubMed]
- Saggar R, Khanna D, Furst DE, et al. Systemic sclerosis and bilateral lung transplantation: a single centre experience. Eur Respir J 2010;36:893-900. [Crossref] [PubMed]
- Schachna L, Medsger TA Jr, Dauber JH, et al. Lung transplantation in scleroderma compared with idiopathic pulmonary fibrosis and idiopathic pulmonary arterial hypertension. Arthritis Rheum 2006;54:3954-61. [Crossref] [PubMed]
- Shitrit D, Amital A, Peled N, et al. Lung transplantation in patients with scleroderma: case series, review of the literature, and criteria for transplantation. Clin Transplant 2009;23:178-83. [Crossref] [PubMed]
- Bernstein EJ, Peterson ER, Sell JL, et al. Survival of adults with systemic sclerosis following lung transplantation: a nationwide cohort study. Arthritis Rheumatol 2015;67:1314-22. [Crossref] [PubMed]
- Savarino E, Bazzica M, Zentilin P, et al. Gastroesophageal reflux and pulmonary fibrosis in scleroderma: a study using pH-impedance monitoring. Am J Respir Crit Care Med 2009;179:408-13. [Crossref] [PubMed]
- D'Ovidio F, Singer LG, Hadjiliadis D, et al. Prevalence of gastroesophageal reflux in end-stage lung disease candidates for lung transplant. Ann Thorac Surg 2005;80:1254-60. [Crossref] [PubMed]
- Raviv Y, D'Ovidio F, Pierre A, et al. Prevalence of gastroparesis before and after lung transplantation and its association with lung allograft outcomes. Clin Transplant 2012;26:133-42. [Crossref] [PubMed]
- Hadjiliadis D, Duane Davis R, Steele MP, et al. Gastroesophageal reflux disease in lung transplant recipients. Clin Transplant 2003;17:363-8. [Crossref] [PubMed]
- Gasper WJ, Sweet MP, Golden JA, et al. Lung transplantation in patients with connective tissue disorders and esophageal dysmotility. Dis Esophagus 2008;21:650-5. [Crossref] [PubMed]
- Patti MG, Gasper WJ, Fisichella PM, et al. Gastroesophageal reflux disease and connective tissue disorders: pathophysiology and implications for treatment. J Gastrointest Surg 2008;12:1900-6. [Crossref] [PubMed]
- Courtwright AM, El-Chemaly S, Dellaripa PF, et al. Survival and outcomes after lung transplantation for non-scleroderma connective tissue-related interstitial lung disease. J Heart Lung Transplant 2017;36:763-9. [Crossref] [PubMed]
- Yazdani A, Singer LG, Strand V, et al. Survival and quality of life in rheumatoid arthritis-associated interstitial lung disease after lung transplantation. J Heart Lung Transplant 2014;33:514-20. [Crossref] [PubMed]
- Ameye H, Ruttens D, Benveniste O, et al. Is lung transplantation a valuable therapeutic option for patients with pulmonary polymyositis? Experiences from the Leuven transplant cohort. Transplant Proc 2014;46:3147-53. [Crossref] [PubMed]


