Sevoflurane did not show better protective effect on endothelial glycocalyx layer compared to propofol during lung resection surgery with one lung ventilation
Introduction
Advances in surgical techniques, anesthetics, anesthetic methods, and critical care have led to a decline in morbidity and mortality after pneumonectomy. However, acute lung injury (ALI) after pneumonectomy is still a dangerous and fatal complication (1) with an incidence ranging between 3% and 10% (2,3).
Factors known to contribute to ALI include surgical trauma, overhydration (3), and one lung ventilation (OLV) (4). In particular, OLV may cause ALI through ventilation-induced injury in the ventilated lung and ischemia-reperfusion injury in the collapsed lung (5).
The endothelial glycocalyx layer (EGL) is a multicomponent layer, comprising glycoprotein and proteoglycan, at the luminal surface of the vascular endothelium (6). EGL plays an important role in tissue fluid balance and edema formation, and has a critical role in maintaining vascular homeostasis (7). EGL injury is an important cause of pulmonary edema during ALI through inflammatory responses, capillary leakage, and edema formation (8). Patients who incur lung injury after pneumonectomy show high pleural fluid protein levels, suggesting they have elevated endothelial permeability, thereby indicating EGL injury.
Despite growing interest on the EGL, no reports have documented EGL injury in relation to lung resection surgery with OLV. Therefore, we aimed to investigate whether lung resection surgery with OLV leads to EGL injury by examining the blood concentrations of EGL components, namely heparan sulfate and human syndecan-1. In addition, we measured the concentrations of vascular cell adhesion molecule-1 (VCAM1) as an indicator of inflammation.
In previous human studies or animal experiments, we measured the damage of EGL resulting from cardiac surgery, trauma patients or transplantation through blood sampling, and the authors also measured this using blood sampling. The most accurate method was to use bronchoalveolar-lavage, but it was not appropriate in patients with pulmonary resection and could not be selected (9-11).
In general, the choice of anesthetics for pneumonectomy has been discussed in relation to hypoxic pulmonary vasoconstriction, but recently, studies have examined the inhibition of inflammatory factors in relation to ALI (12). Recent studies have reported that sevoflurane, an inhalational anesthetic, protects the EGL from ischemia-reperfusion injury (13) by reducing the adhesion of leukocytes and platelets to the vascular endothelium (14). Another mechanism seems to sevoflurane attenuate lysosomal cathepsin B releasing and to be independent from tissue mast cell degranulation (15). Hence, we investigated whether sevoflurane was superior to propofol in reducing EGL injury during lung resection surgery with OLV.
Our hypothesis was that lung resection surgery with OLV produces EGL damage and sevoflurane protects the EGL better than the intravenous anesthetic propofol does.
Methods
Patient selection
This study was approved by the hospital’s institutional ethics committee (No. PNUYH 05-2014-098), and informed consents were obtained from the patients the day before surgery. Eighty-seven patients with American Anesthesiology Association physical status 1 and 2 who were scheduled to undergo lung resection surgery with OLV were enrolled in this study. Patients who had a history of surgery for cardiopulmonary disease, patients who had current severe cardiopulmonary disease, patients with diabetes mellitus, patients with renal dysfunction, and current smokers were excluded from this study. The OLV time within 120 minutes was excluded because it predicted EGL damage was less and if more than 500 mL of bleeding that could lead to transfusion was also exclude. The participants were randomly divided into Group S (sevoflurane anesthesia; n=43) or Group P (propofol anesthesia; n=44) by using a computer-generated randomization table.
Anesthetic management and surgery
All patients fasted since midnight the day of surgery and received intramuscular injection of glycopyrrolate (0.2 mg) before being transferred to the operating room. Upon arrival at the operating room, the patients were attached to electrocardiography, non-invasive blood pressure, and pulse oximetry monitors. Prior to the induction of general anesthesia, a mid-thoracic epidural catheter was placed at T5/6 on all patients for postoperative pain control, and a test dose (3 mL of 2% lidocaine +0.015 mg epinephrine) was injected through the epidural catheter to confirm subarachnoid or intravascular injection. Then, 5 mg/kg thiopental followed by 0.8 mg/kg rocuronium and fentanyl 50–100 µg were intravenously injected to induce anesthesia, after which a double-lumen endobronchial tube (Broncho-Cath; Mallinckrodt Laboratories, Athlone, Ireland) was inserted through the airway. The position of the endobronchial tube was confirmed using fiberoptic bronchoscopy. A catheter was placed in the radial artery for invasive arterial pressure monitoring, and a central venous catheter was placed in the subclavian vein. The patient was positioned in the lateral decubitus position to reveal the surgical site, which was disinfected before beginning OLV (4–6 mL/kg tidal volume guaranteed pressure ventilation +5 cmH2O positive end-expiratory pressure). Intraoperative end-tidal CO2 was maintained at 35–45 mmHg, and peak inspiratory pressure was controlled not to exceed 30 cmH2O.
After position changed to lateral decubitus position, fentanyl 10 µg/mL in levobupivacaine 0.125% was administered epidurally as a bolus 6 mL, followed by continuous infusion of 2 mL/h.
Group S received 1.5–2.5 vol% sevoflurane, and group P received propofol at 50–150 µg/kg/min to maintain the bispectral index at 40–60. Plasma-lyte (Plasma solution A Inj®; CJ HealthCare, Korea) was used for intraoperative fluid management.
Blood sampling
Blood samples were drawn from all patients immediately after the induction of general anesthesia for baseline measurement (T1). Blood samples were also drawn 60 min after OLV (T2), 120 min after OLV (T3), end of OLV (T4), and after skin suturing (T5). According to previous studies, all blood samples were drawn from patient’s radial artery catheter (10,16).
Heparan sulfate, human syndecan-1, and VCAM1 concentration measurement
Arterial blood samples were drawn into ethylenediaminetetraacetic acid tubes and immediately centrifuged at 1,000 ×g for 10 min; the supernatant was then removed and centrifuged for an additional 3 min at 7,000 ×g. The plasma was frozen at −80 °C until subsequent analysis. Heparan sulfate, human syndecan-1, and VCAM1 concentrations were analyzed using specific enzyme immunoassay kits (Syndecan-1: Abcam, Cat. No. ab46506, Cambridge, MA, USA; heparan sulfate: Biotang, Cat. No. HU8718, Lexington, MA, USA; VCAM1: Abcam, Cat. No. ab187393, Cambridge, MA, USA). All samples were tested in duplicate. Samples that were over the detection range of the assay were diluted and rerun as needed.
Statistical analysis
All statistical computations were performed using IBM SPSS version 22 (IBM, USA). All variables were presented as mean ± standard deviation. Dichotomous variables such as operation type were compared using Fisher’s exact test. Basal measurement (T1) and measurements at T2–T5 were compared using the repeated measures ANOVA. Group S and Group P were compared at each time point by using the Wilcoxon signed-rank test, and statistical significance was set at a P value <0.05.
Results
Demographic data and procedural characteristics
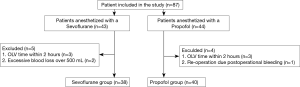
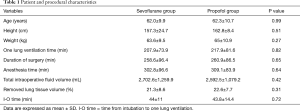
From among the 87 patients, nine were excluded, specifically for receiving OLV within 120 minutes (n=6), excessive blood loss during surgery (n=2), and re-surgery due to bleeding (n=1) (Figure 1). The patient’s demographic data and procedural characteristics are shown in Table 1. No statistical differences were observed between the two groups in their demographic data. Further, no differences were observed in OLV time, total length of surgery, duration of anesthesia, and fluid volume, which were factors that could affect the outcome.
Full table
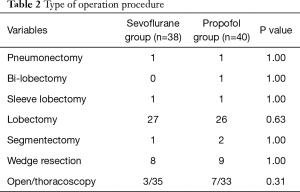
Surgical techniques and types used for the patients are shown in Table 2. No significant differences were observed between the two groups. All surgeries were performed by two thoracic surgeons.
Full table
Heparan sulfate, human syndecan-1, and VCAM1
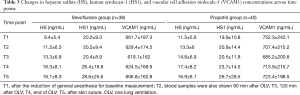
Changes in heparan sulfate, human syndecan-1, and VCAM1 concentrations across time points are shown in Table 3.
Full table
Heparan sulfate concentrations began to increase after beginning OLV and significantly increased from 120 min after OLV (T3) to the end of surgery in both groups (P<0.05). However, there was no statistically significant difference between the both group (P>0.05) (Figure 2). Group P showed a higher heparan sulfate concentration than Group S at all-time points, but without significant intergroup differences (Figure 3).
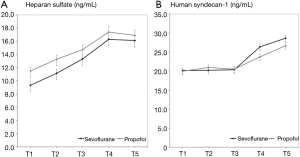
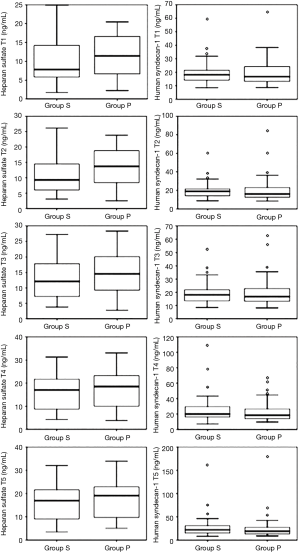
Human syndecan-1 concentration began to increase about end of OLV (T4) in both groups, and it was significantly elevated at the end of surgery (T5) only in Group S, but there was no statistically significant difference between the both group (P>0.05) (Figure 2). No significant intergroup differences in human syndecan-1 concentration were observed at any of the time points from T1 to T5 (Figure 3).
VCAM1 concentration did not increase above the basal level in either group (Table 3).
Discussion
In this study, lung resection surgery with OLV was temporally correlated EGL damage that was not related to any inflammatory reaction. Compared to propofol, sevoflurane did not show a better protective effect on the EGL.
OLV, which is performed for lung resection surgery is known to be associated with postoperative pulmonary complications and has been reported as a risk factor for ALI (4). The underlying mechanism for OLV-induced ALI involves barotrauma and hyperperfusion/capillary shear stress in the dependent lung and atelectasis-recruitment, ischemia-reperfusion injury, and surgical trauma in the non-dependent lung (5). An injury to the EGL, which forms the inner wall of the alveolar-capillary membrane, plays an important role in such lung injuries (17). Thus, we measured the concentrations of the main components of EGL, namely heparan sulfate and human syndecan-1, in patients undergoing OLV for lung resection surgery, and compared the concentrations between patients receiving sevoflurane, an inhalational anesthetic, and propofol, an intravenous anesthetic.
In patients undergoing lung resection surgery, the concentrations of heparan sulfate increased with increasing duration of OLV. This is in line with the prevalent knowledge that OLV is a risk factor of lung injury. Further, this supports Licker et al.’s (18) finding that the incidence of ALI increases with increasing area of resection, as it is related to lengthening the duration of OLV.
Heparan sulfate concentration significantly increased from 120 min after beginning OLV in both Group S and Group P, but human syndecan-1 concentration was significantly elevated after skin suturing in Group S. Human syndecan-1 is a core protein strongly bound to the blood vessels, and heparan sulfate is a side chain attached to it. When the EGL is destroyed, the side chain is believed temporally injured before or injured more severely than the core protein attached to the vessels (19).
According to Annecke et al. (15), sevoflurane exerts some protective effects on the EGL against ischemia-reperfusion injury, but in our study, sevoflurane did not significantly decrease the concentrations of EGL damage markers heparan sulfate and human syndecan-1 than did propofol (control). In our experiment, lung resection surgery with OLV was performed for an adequate amount of time (mean duration, 300 min), and the markers were measured at various time points from immediately after induction to 60 min after OLV, 120 min after OLV, end of OLV, and end of surgery, thereby lowering the possibility of inadequate OLV duration or measurement errors. One possible reason for the finding that sevoflurane was ineffective in protecting the EGL, unlike in previous studies, may be that this study included different study subjects and methods. Similar to our study, Annecke et al. (15) measured heparan sulfate and syndecan-1 concentrations to determine the presence of EGL injury, but they studied guinea pigs and the heart, as opposed to the lungs in our study.
The collapsed lung is known to incur major damage from ischemia-reperfusion injury during OLV, but the alveolar damage caused by OLV has been reported to occur because of hyperperfusion and hyperinflation of the ventilated lung (20). Because we measured heparan sulfate and human syndecan-1 concentrations in both lungs, we do not believe that it shows the effects of sevoflurane on ischemia-reperfusion injury.
We measured blood VCAM1 concentration to examine whether EGL damage was associated with inflammatory reactions. VCAM1 regulates leukocyte recruitment during an inflammatory reaction, and plays an important role in inducing lung injury (21). In a histological analysis in animals, Kozian et al. (20) reported that the ventilated lung shows alveolar edema, interstitial edema, microhemorrhage, and neutrophil infiltration 90 min after OLV. Schilling et al. reported that sevoflurane and desflurane induce fewer proinflammatory responses than does propofol during OLV (12,22). However, in our study, the concentration of the inflammatory marker VCAM1 was not elevated from the baseline (immediately after induction) across any of the time points (60 min after OLV, 120 min after OLV, end of OLV, and end of surgery). Unlike our study, previous studies measured inflammation by using the concentrations of tumor necrosis factor-alpha (TNF-α) and interleukin (IL) via bronchoalveolar lavage. However, VCAM1 is known to be upregulated in response to TNF-α and Interleukin. The lack of differences in VCAM1 concentrations between the two groups may be attributable to the short blood sampling period. Another reason may be that VCAM1 concentrations are not elevated concurrently with an elevation of heparan sulfate and human syndecan-1 concentrations, suggesting that EGL damage may occur independently of inflammatory reactions.
This study has a few limitations. First, we measured EGL damage in the collapsed, non-dependent lung and ventilated, dependent lung simultaneously. Different outcomes may have been produced if we had performed separate measurements, i.e., if we had measured EGL damage caused by ischemia-reperfusion in the non-dependent lung and EGL damage caused by hyperperfusion-hyperventilation in the dependent lung. In the future, studies that measure EGL damage markers from lavage fluids taken from each lung through bronchoalveolar lavage may be helpful. Second, thoracic epidural analgesia may have reduced the degree of overall inflammatory responses. According to Enigk et al. (23), thoracic epidural anesthesia lowers endothelial injury by suppressing the expression of IL-1β and adhesion molecule by endotoxin and inhibiting leukocyte adhesion. Further, the thoracic analgesia performed in our study may have inhibited the overall inflammatory reactions and subsequent reductions of EGL damage may have contributed to the lack of significant differences between the two groups. Third, the study population was limited to patients undergoing lung resection with a minimum of 2 hours of OLV. Considering that human syndecan-1 concentrations tend to show no changes until 2 hours of OLV and begin to increase only after 2 hours, a longer observation of the EGL markers may have led to different findings. Finally, we failed to shed light on the association between an elevation of heparan sulfate and human syndecan-1 concentrations with postoperative complications. Complications were not the primary endpoint of our study, and future studies should include a greater sample size to examine complications and draw meaningful conclusions.
In conclusion, lung resection surgery with OLV produced duration-dependent EGL damage that was measured using the concentrations of plasma heparan sulfate and human syndecan-1. Sevoflurane, which is known for its protective effect against ischemia-reperfusion injury, however, did not show any more beneficial effect on EGL than did the intravenous anesthetic propofol.
Acknowledgements
We acknowledge the assistance of Pusan National University School of Medicine, Research Institute for Convergence Biomedical Science and Technology Pusan National University Yangsan Hospital.
Funding: This study was supported by Research Institute for Convergence of Biomedical Science and Technology (No. 30-21018-0018) Pusan National University Yangsan Hospital.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the hospital’s institutional ethics committee (No. PNUYH 05-2014-098), and informed consents were obtained from the patients the day before surgery.
References
- Watanabe S, Asamura H, Suzuki K, et al. Recent results of postoperative mortality for surgical resections in lung cancer. Ann Thorac Surg 2004;78:999-1002; discussion 1002-3. [Crossref] [PubMed]
- Turnage WS, Lunn JJ. Postpneumonectomy pulmonary edema. A retrospective analysis of associated variables. Chest 1993;103:1646-50. [Crossref] [PubMed]
- Alam N, Park BJ, Wilton A, et al. Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg 2007;84:1085-91. [Crossref] [PubMed]
- Lohser J. Evidence-based management of one-lung ventilation. Anesthesiol Clin 2008;26:241-72. [Crossref] [PubMed]
- Lohser J, Slinger P. Lung Injury after One-Lung Ventilation: A Review of the Pathophysiologic Mechanisms Affecting the Ventilated and the Collapsed Lung. Anesth Analg 2015;121:302-18. [Crossref] [PubMed]
- van Haaren PM, VanBavel E, Vink H, et al. Localization of the permeability barrier to solutes in isolated arteries by confocal microscopy. Am J Physiol Heart Circ Physiol 2003;285:H2848-56. [Crossref] [PubMed]
- Adamson RH, Lenz JF, Zhang X, et al. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol 2004;557:889-907. [Crossref] [PubMed]
- Collins SR, Blank RS, Deatherage LS, et al. Special article: the endothelial glycocalyx: emerging concepts in pulmonary edema and acute lung injury. Anesth Analg 2013;117:664-74. [Crossref] [PubMed]
- Rahbar E, Cardenas JC, Baimukanova G, et al. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med 2015;13:117. [Crossref] [PubMed]
- Rehm M, Bruegger D, Christ F, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 2007;116:1896-906. [Crossref] [PubMed]
- Casanova J, Simon C, Vara E, et al. Sevoflurane anesthetic preconditioning protects the lung endothelial glycocalyx from ischemia reperfusion injury in an experimental lung autotransplant model. J Anesth 2016;30:755-62. [Crossref] [PubMed]
- Schilling T, Kozian A, Senturk M, et al. Effects of volatile and intravenous anesthesia on the alveolar and systemic inflammatory response in thoracic surgical patients. Anesthesiology 2011;115:65-74. [Crossref] [PubMed]
- Becker BF, Chappell D, Bruegger D, et al. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res 2010;87:300-10. [Crossref] [PubMed]
- Chappell D, Heindl B, Jacob M, et al. Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology 2011;115:483-91. [Crossref] [PubMed]
- Annecke T, Chappell D, Chen C, et al. Sevoflurane preserves the endothelial glycocalyx against ischaemia–reperfusion injury. Br J Anaesth 2010;104:414-21. [Crossref] [PubMed]
- Annecke T, Rhem M, Bruegger D, et al. Ischemia-reperfusion-induced unmeasured anion generation and glycocalyx shedding: sevoflurane versus propofol anesthesia. J Invest Surg 2012;25:162-8. [Crossref] [PubMed]
- Schmidt EP, Li G, Li L, et al. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J Biol Chem 2014;289:8194-202. [Crossref] [PubMed]
- Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg 2006;81:1830-7. [Crossref] [PubMed]
- Reitsma S, Slaaf DW, Vink H, et al. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 2007;454:345-59. [Crossref] [PubMed]
- Kozian A, Schilling T, Freden F, et al. One-lung ventilation induces hyperperfusion and alveolar damage in the ventilated lung: an experimental study. Br J Anaesth 2008;100:549-59. [Crossref] [PubMed]
- Mishra A, Guo Y, Zhang L, et al. A Critical Role for P2X7 Receptor-Induced VCAM-1 Shedding and Neutrophil Infiltration during Acute Lung Injury. J Immunol 2016;197:2828-37. [Crossref] [PubMed]
- Schilling T, Kozian A, Kretzschmar M, et al. Effects of propofol and desflurane anaesthesia on the alveolar inflammatory response to one-lung ventilation. Br J Anaesth 2007;99:368-75. [Crossref] [PubMed]
- Enigk F, Wagner A, Samapati R, et al. Thoracic epidural anesthesia decreases endotoxin-induced endothelial injury. BMC Anesthesiol 2014;14:23. [Crossref] [PubMed]