Veno-venous extracorporeal membrane oxygenation for the third millennium
Veno-venous extracorporeal membrane oxygenation (VV-ECMO) can now be considered as a rescue therapy for severe acute respiratory distress syndrome (ARDS) patients when mechanical ventilation is not sufficient to ensure efficient oxygenation and decarboxylation, or when high intrathoracic pressure are required to maintain adequate gas exchange (1). This support now allows for an “ultra”-protective mechanical ventilation, and the control of intra-thoracic pressure, which reduces the risk of lung injury (2,3). However, these ECMO-treated ARDS patients still exhibited a high mortality, and the technique is still marred by numerous complications. Mortality has been reported from 21% to 43% (2-5), and 40% of patients developed at least one complication where bleeding is the most frequent (6). Nevertheless, it is worth considering the technical breakthrough of devices obtained for 2 decades. ECMO equipment are now more biocompatible and offer more effective gas exchange with lower flow resistance (7). In addition, it is likely that the experience gained by ECMO-referral centers, with a better identification of ECMO-indications and timing, will contribute to improve outcomes in a near future. Meanwhile, a large number of ECMO management aspects are still unclear and deserve focus of clinicians in the next years.
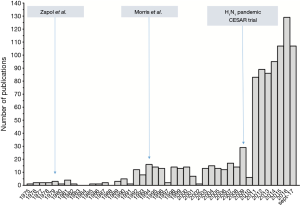
Although the numbers of VV-ECMO use and studies on this topic have both steadily increased since 2009, research is still limited to monocenter (8,9) or retrospective cohorts (4,5) that preclude providing strong evidence to guide clinician therapeutic strategy (Figure 1). In other words, international randomized controlled trials (RCTs) on modern ECMO for ARDS are urgently needed to answer several controversial management points (12) that have not been fully clarified with the CESAR trial (3). Timing of ECMO implantation, and patient selection are the first burning points that RCTs should answer as early ECMO initiation with limitation of ventilator-induced lung injury is still balanced with inherent complications of cannulation and anticoagulation, which may limit its clinical benefits. Results of the ongoing international multicenter randomized Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA, NCT01470703), compared early ECMO initiation (≤7 days on mechanical ventilation) with conventional ventilation management strategy in patients with severe-ARDS, is highly expected. It may contribute to clarifying the place of ECMO in the management of severe ARDS. Selection of the patients who are more likely to benefit from that technique is also a matter of concern. For instance, no change in mortality was reported in a large German cohort, between the year 2007 and 2014, where the proportion of patients older than 80 years on VV-ECMO increased over time (13). The extension of this invasive and expensive technique to older aged patients is highly questionable, as mortality is already high even when it is restricted to young patients. Moreover, their overall mortality was higher than reported in other multicenter studies (5) with mortality rates of patients on ECMO for less than 48 hours reached 70%. This aspect highlights potential procedure-related complications and patient selection issues. In order to improve patient selection for ECMO, some survival predictive models have been developed (4,5,8,9). These scores deserve external validation in international studies, where ECMO has not yet been instituted. It may provide a relevant tool for clinicians to selecting the best candidates for ECMO in the light of the risk-benefit balance.
Anticoagulation and drugs’ pharmacokinetics on ECMO need investigation as well. Circuits are now made with more biocompatible materials and a hollow-fiber oxygenator. Thus, a decrease of platelets and plasma protein consumption with these new circuits could allow a reduction of anticoagulation on ECMO (14). Moreover, numerous published case reports of successful management on prolonged VV-ECMO with no anticoagulation have been recently reported (15). Whether a systematic reduction of anticoagulation on VV-ECMO could be safely applied without increasing venous thrombosis and oxygenator clotting is still undetermined and requires a RCT to evaluate risks and benefits of each strategy. The ECMO circuit itself, and the increase of the distribution volume, strongly impact antibiotics’ pharmacokinetic and pharmacodynamics, which can lead to toxicity or therapeutic failure (16,17). The future of ECMO patient care should consider the pharmacological properties of the drug, and its kinetics on ECMO to provide a daily personalized titration (18). Similarly, mechanical ventilation on ECMO should be investigated as well. First, the benefit of “ultra”-protective ventilation, which associates very low tidal volume (19), low driving pressure (20) and a high positive end-expiratory pressure (PEEP) (21) deserve to be confirmed on a large ECMO population. For instance, application of a high PEEP level, with reduction of the driving pressure, will depend on the distribution of the ARDS lesions and the morphology of the patient. Then, “optimal” PEEP could be very different between patients and during the course of ARDS, suggesting individualizing mechanical ventilation settings with bedside tools. For example, Franchineau et al. recently suggested that electrical impedance tomography could contribute to set PEEP on ECMO with a bedside monitoring of atelectasis and overdistension (22). Similarly, the use of esophageal pressure or pressure-volume curve to personalize mechanical ventilation on ECMO warrants investigation.
The ability of ECMO to fully replace lung function, even in severe ARDS and the toxicity of mechanical ventilation, may question the reasons to maintain mechanical ventilation on ECMO. A strategy of non-invasive mechanical ventilation with ECMO for severe ARDS has been reported in an uncontrolled pilot trial of six patients (23). Half of the patients died on ECMO, whereas others were successfully discharged from the hospital. In addition, this strategy allows the use of less sedative and to maintain oral feeding, muscular activity and social interaction. In a different population, an ECMO strategy without mechanical ventilation before a lung transplant was associated to a better 6-month post-transplant survival when compared to a strategy based on mechanical ventilation only (24,25). Better understanding of the respiratory drive determinants on ECMO are urgently required to develop this strategy as inappropriate spontaneous ventilation in ARDS could also worsen lung injuries and compromise clinical outcomes (26-28). Because ECMO may run for several weeks and psychological and physical issues mar long-term quality of life of ARDS survivors, early rehabilitation and mobilization (even) on ECMO must be a priority. Abrams et al. recently reported that active physical therapy on VV-ECMO could be safely performed (29). Positive effects of early mobilization on physical status at hospital discharge, muscle strength and long-term outcome (30) should encourage clinicians to implement this strategy with our ECMO patients. Lastly, regional organization of ECMO activity is a major challenge. Expanding utilization of ECMO during past decades has led to increase the number of centers performing ECMO, especially Veno-venous. Important disparities regarding the number of cases per center was noted with higher annual hospital ECMO volume associated with lower mortality (31). The recent report of German ECMO activity has stressed out the limits of a system without ECMO network (13). The lack of regional and/or national organization with identified ECMO referral centers led to an unexpected high mortality (58%) with an inverse relationship between annual ECMO case volume and mortality with VV-ECMO (13). This data calls for performing ECMO in expert referral centers with a 24/7 mobile ECMO team inside a regional and/or national network. In addition to improving patients’ outcome, this strategy may also lead to a better cost-effectiveness balance (32).
Since 2009, we have seen a strong regain of interest for ECMO in refractory severe ARDS. The “rebirth” of this technique, after encouraging results obtained during the H1N1 pandemic and in the CESAR trial, must not hide the fact that our knowledge of its risk-benefit ratio and its daily management are actually limited. Future research and results of the EOLIA trial are highly expected to start answering to the (still) burning question of “When, to whom, and how to perform ECMO for severe ARDS?”
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011;365:1905-14. [Crossref] [PubMed]
- Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009;302:1888-95. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med 2014;189:1374-82. [Crossref] [PubMed]
- Schmidt M, Zogheib E, Roze H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013;39:1704-13. [Crossref] [PubMed]
- Vaquer S, de Haro C, Peruga P, et al. Systematic review and meta-analysis of complications and mortality of veno-venous extracorporeal membrane oxygenation for refractory acute respiratory distress syndrome. Ann Intensive Care 2017;7:51. [Crossref] [PubMed]
- Lehle K, Philipp A, Hiller KA, et al. Efficiency of gas transfer in venovenous extracorporeal membrane oxygenation: analysis of 317 cases with four different ECMO systems. Intensive Care Med 2014;40:1870-7. [Crossref] [PubMed]
- Enger T, Philipp A, Videm V, et al. Prediction of mortality in adult patients with severe acute lung failure receiving veno-venous extracorporeal membrane oxygenation: a prospective observational study. Crit Care 2014;18:R67. [Crossref] [PubMed]
- Roch A, Hraiech S, Masson E, et al. Outcome of acute respiratory distress syndrome patients treated with extracorporeal membrane oxygenation and brought to a referral center. Intensive Care Med 2014;40:74-83. [Crossref] [PubMed]
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979;242:2193-6. [Crossref] [PubMed]
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994;149:295-305. [Crossref] [PubMed]
- Combes A, Brodie D, Chen YS, et al. The ICM research agenda on extracorporeal life support. Intensive Care Med 2017;43:1306-18. [Crossref] [PubMed]
- Karagiannidis C, Brodie D, Strassmann S, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med 2016;42:889-96. [Crossref] [PubMed]
- MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 2012;38:210-20. [Crossref] [PubMed]
- Herbert DG, Buscher H, Nair P. Prolonged venovenous extracorporeal membrane oxygenation without anticoagulation: a case of Goodpasture syndrome-related pulmonary haemorrhage. Crit Care Resusc 2014;16:69-72. [PubMed]
- Shekar K, Fraser JF, Smith MT, et al. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care 2012;27:741.e9-18. [Crossref] [PubMed]
- Shekar K, Roberts JA, McDonald CI, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care 2012;16:R194. [Crossref] [PubMed]
- Shekar K, Roberts JA, Smith MT, et al. The ECMO PK Project: an incremental research approach to advance understanding of the pharmacokinetic alterations and improve patient outcomes during extracorporeal membrane oxygenation. BMC Anesthesiol 2013;13:7. [Crossref] [PubMed]
- Bein T, Weber-Carstens S, Goldmann A, et al. Lower tidal volume strategy (approximately 3 ml/kg) combined with extracorporeal CO2 removal versus 'conventional' protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Medicine 2013;39:847-56. [Crossref] [PubMed]
- Serpa Neto A, Schmidt M, Azevedo LC, et al. Associations between ventilator settings during extracorporeal membrane oxygenation for refractory hypoxemia and outcome in patients with acute respiratory distress syndrome: a pooled individual patient data analysis: Mechanical ventilation during ECMO. Intensive Care Med 2016;42:1672-84. [Crossref] [PubMed]
- Schmidt M, Stewart C, Bailey M, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: a retrospective international multicenter study. Crit Care Med 2015;43:654-64. [Crossref] [PubMed]
- Franchineau G, Bréchot N, Lebreton G, et al. Bedside Contribution of Electrical Impedance Tomography to Setting Positive End-Expiratory Pressure for Extracorporeal Membrane Oxygenation-treated Patients with Severe Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2017;196:447-57. [Crossref] [PubMed]
- Hoeper MM, Wiesner O, Hadem J, et al. Extracorporeal membrane oxygenation instead of invasive mechanical ventilation in patients with acute respiratory distress syndrome. Intensive Care Med 2013;39:2056-7. [Crossref] [PubMed]
- Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012;185:763-8. [Crossref] [PubMed]
- Trudzinski FC, Kaestner F, Schafers HJ, et al. Outcome of Patients with Interstitial Lung Disease Treated with Extracorporeal Membrane Oxygenation for Acute Respiratory Failure. Am J Respir Crit Care Med 2016;193:527-33. [Crossref] [PubMed]
- Yoshida T, Torsani V, Gomes S, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med 2013;188:1420-7. [Crossref] [PubMed]
- Yoshida T, Roldan R, Beraldo MA, et al. Spontaneous Effort During Mechanical Ventilation: Maximal Injury With Less Positive End-Expiratory Pressure. Crit Care Med 2016;44:e678-88. [Crossref] [PubMed]
- Yoshida T, Nakahashi S, Nakamura MAM, et al. Volume-controlled Ventilation Does Not Prevent Injurious Inflation during Spontaneous Effort. Am J Respir Crit Care Med 2017;196:590-601. [Crossref] [PubMed]
- Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 2014;63:2769-78. [Crossref] [PubMed]
- Tipping CJ, Harrold M, Holland A, et al. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med 2017;43:171-83. [Crossref] [PubMed]
- Barbaro RP, Odetola FO, Kidwell KM, et al. Association of Hospital-Level Volume of Extracorporeal Membrane Oxygenation Cases and Mortality - Analysis of the Extracorporeal Life Support Organization Registry. Am J Respir Crit Care Med 2015;191:894-901. [Crossref] [PubMed]
- Combes A, Brodie D, Bartlett R, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med 2014;190:488-96. [Crossref] [PubMed]


