Expression of TGF-beta receptor 1 and Smads in the tissues of primary spontaneous pneumothorax
Introduction
The incidence of primary spontaneous pneumothorax (PSP) was 7.4–18/100,000/year for males and 1.2–6/100,000/year for females according to the global statistical data (1,2). PSP usually presents as an acute onset and has high recurrence rate, so that brings a strong attack on the quality of patient’s life and healthy economy of the community. Therefore, it is important to explore the pathogenesis of PSP. Unfortunately, no basal pulmonary disease is found in majority of the PSP cases though it is a popular disease especially in younger populations. So the causes and pathogenesis of PSP have not yet been clarified till now. Some previous reports have shown that there is fibrosis in addition to the bullae in the apex of the lung in young PSP subjects (3,4). According to studies of histopathology, the histopathologic feature of PSP lesion is alveolar collapse accompanied by fibroelastosis. And these pathological findings are not secondary to pneumothorax but have existed before the episode of pneumothorax. One hypothesis based on the results of Kobayashi et al. is that as thickened pleura with fibroelastosis becomes less compliant, and pleural thickening is uneven, areas of thinner apical visceral pleura, which may occasionally be at the site of subpleural bullae, receive a higher negative pressure and thus become likely to rupture (5). The presence of inflammatory cells and occasional fibrosis in the lesion regions which altered the delicate structures of elastic fibers in the lung, suggested that inflammation or pulmonary fibrosis might play a role in the aberrant changes of PSP.
TGF-β1 and its downstream cytokines Smad families are related with pulmonary fibrosis according to previous literatures (6). Moreover, it is reported by Sibinska and some other researchers that activation of interstitial lung fibroblast and presence of extracellular matrix production are pathological features of pulmonary fibrosis, and the effect of TGF-β1 on the pathological changes is mainly mediated through the TGF-β/Smads signaling pathway. Moreover, the anti-fibrosis effect of heat shock protein HSP90 is related to TGF-β receptor destruction and inhibition of Smad2/3 activation (7,8). So, it is thought that the role of TGF-β and its downstream cytokines in PSP bullae might induce pulmonary fibrosis. In the TGF-β/Smads pathway, TβR is a receptor protein with high affinity for TGFβ on the surface of cell membrane. Smad2, Smad3 and Smad4 play an important role in conducting signal into the nucleus and regulating transcription. Our study aimed to these cytokines associated with pulmonary fibrosis. The expression of TGF-beta receptor 1 (TβR1), Smad2, Smad3 and Smad4 in either the bullae tissue or normal lung tissue were tested to explore whether there was correlation between fibrosis relevant factors and pulmonary bullae disease.
Methods
Participant samples
From May 2015 to May 2016, 34 consecutive PSP patients who underwent surgery treatment at the Department of Thoracic Surgery, Beijing Chao-Yang Hospital, and met our inclusion criteria were enrolled as the study group in this study. PSP was defined as spontaneous air accumulation in the pleural cavity without other evidence of clinical lung disease. The inclusion criteria were: (I) all cases were in accordance with PSP diagnostic criteria—the lungs of PSP patients were no other lesions, confirmed by chest imaging diagnosis; and (II) patient age between 15 and 40 years. The exclusion criteria were: (I) history of smoking; and (II) a history of chest trauma, rib fracture and pulmonary contusion; (III) a history of pulmonary surgery, including lobectomy, segmentectomy and wedge resection of the lung; and (IV) a history of lung diseases (i.e., chronic obstructive pulmonary disease (COPD), asthma, pulmonary tuberculosis, pneumonia or pneumoconiosis); (V) a history of systemic diseases (i.e., end-stage renal failure, liver cirrhosis, malignancy, or chronic heart and liver diseases). At the same time, 10 patients without pneumothorax associated disease were selected as the control group. The cases of the control group were selected from the same age-bracket. There are 4 patients with lung cancer, 3 patients with bronchiectasis, 2 patients with tuberculosis and 1 patient with lung abscess enrolled in the control group. All patients of them underwent thoracoscopic lobectomy or partial lobectomy. The tissues of the control group were obtained far away from the site of the primary lesions, and identified by two pathologists as normal lung tissue. The age, sex, body mass index (BMI, kg/m2), recurrence of pneumothorax and side of pneumothorax were recorded (Table 1). The institutional research ethics committee of the Beijing Chaoyang Hospital approved the study, and all of the patients signed the informed consent preoperatively.
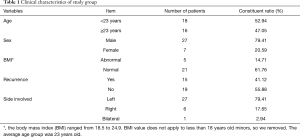
Full table
Immunohistochemistry
Expression of TβR1, Smad2, Smad3 and Smad4 in the pathological sections was assessed by an Immunohistochemical SP method staining. We purchased the primary antibodies from the Abcam Company (Cambridge, UK). Anti-TGF beta Receptor I antibody (rabbit polyclonal to TGF beta receptor I, ab31013), anti-Smad2 antibody (rabbit monoclonal to Smad2, ab40855), Anti-Smad3 antibody (rabbit monoclonal to Smad3, ab40854) and anti-Smad4 antibody (rabbit monoclonal to Smad4, ab40759). Murine anti-rabbit polyclonal antibody was selected as the secondary antibody. Paraffin blocks were sectioned continuously at a thickness of 4 µm for each case. The sections were deparaffinized in xylene. After the xylene was subsequently removed with absolute ethanol, the slides were treated with 3% H2O2 which can inactivate endogenous peroxidase. The sections were incubated with the antibodies to TβR1, Smad2, Smad3 and Smad4 respectively. The reaction was followed by biotin-conjugated rabbit anti-human immunoglobulin and horseradish peroxidase-conjugated streptavidin. Diaminobenzidine was used as chromogenic substrate, and brown or crimson red precipitates were identified as positive staining. The slides were counterstained with hematoxylin and mounted with glycerol gelatin. In each experiment, a section of PSP known to express TβR1, Smad2, Smad3 and Smad4 served as a positive control, Negative control was performed in the same way without addition of the primary antibodies.
Slide evaluation
Each slide was evaluated randomly in five areas with obvious lesions. Photographs were taken at each evaluated area for records. Each slide was read and scored by two independent investigators. Immunoreactivity was evaluated using the staining intensity score and distribution score. The Immunoreactivity was scored semi-quantitatively on the basis of a well-established immunoreactivity scoring system (IRS) (9-11). The IRS is calculated by the product of the percentage of positive cells (>80%, 4 points; 51–80%, 3 points; 11–50%, 2 points; 0–10%, 1 point; 0%, 0 point) and the intensity of the staining (strong, 3 points; moderate, 2 points; mild, 1 point; and no staining, 0 point). The final scores are between 0 (no staining) and 12 (maximum staining). The IRS were divided into one of the following three groups based on the final score; negative immunoreactivity was defined as a total score of 0, low expression was defined as a total score of 1–4, and high expression was defined as a total score of >4.
Statistical analysis
The statistical analyses were conducted using SPSS software (version 17.0; SPSS Inc., Chicago, III, USA). The continuous variables are presented as the mean ± standard deviation. Statistical analyses to compare two groups of data were performed using an unpaired Student’s t-test. Ratio analysis was performed with Fisher’s exact test. Correlations between low-expression and high-expression groups of TβR1, Smad2, Smad3 and Smad4 with age, sex, BMI, recurrence and side of pneumothorax were analyzed using the chi-square test or Fisher’s exact test. P values less than 0.05 were considered statistically significant.
Results
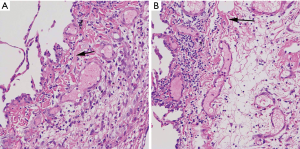
Pulmonary bulla combined with fibrotic representation in H&E staining
By H&E staining of pulmonary bullae tissue sections, we found a large number of fibrous tissue around the bulla tissue. It suggested that there was frequent representation of pulmonary fibrosis in pulmonary bullae tissue (Figure 1).
The expression of TβR1, Smad2, Smad3 and Smad4 at the site of bullae tissue and normal tissues from the same PSP patients
To investigate the expression levels of TβR1, Smad2, Smad3 and Smad4 both in the bullae tissue and in normal tissues of the same PSP patients, the immunohistochemistry staining was performed in specimens of 16 cases. Observing through microscope, the expression of TβR1, Smad2 and Smad4 in the PSP bullae tissue were obviously concentrated staining. We found that TβR1, Smad2 and Smad4 mainly expressed in the alveolar type II cell. TβR1 mostly expressed in the membrane, while a tiny portion of its expression can be observed in the cytoplasm. Smad2 and Smad4 mostly expressed in the cytoplasm as well as in the nucleus. In the contrary, the normal lung tissue around the bullae in the same section showed less obvious staining (Figure 2).
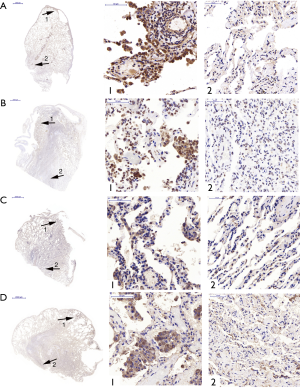
The results of Fisher exactness analysis showed that the expression levels of TβR1, Smad2 and Smad4 in pulmonary bullae were significantly higher than that in normal lung tissue (P=0.001, 0.023, 0.015 respectively) (Table 2). These results suggested that TβR1, Smad2 and Smad4 highly expressed in the bullae area of patients with PSP. The expression of Smad3 in pulmonary bullae was not showed significantly different from that in normal lung tissues (P=0.172) (Table 2). But observation through microscope showed that the coloration intensity and density of Smad3 in the bullae tissue was obviously higher than that in the normal tissues around the bullae. Smad3 mostly expressed in the cytoplasm and the nucleus (Figure 2).

Full table
Expression of TβR1, Smad2, Smad3 and Smad4 in lung tissue of PSP patients and control group
There were 34 PSP patients included as the study group. And 10 patients without pneumothorax associated disease were selected as the control group. The constituent ratio of TβR1, Smad2, samd3 and Smad4 semi-quantitative score reaching “++” of the study group were significantly higher than that of the control group (Table 3).

Full table
The IRS scores of TβR1, Samd2 and Smad4 of the study group were significantly higher than that of the control group, and the differences were statistically significant (P=0.012, 0.031, 0.000 respectively) (Table 4). Observing through microscope, the expression of TβR1, Smad2 and Smad4 in the PSP bullae tissue were obviously concentrated staining. We found that TβR1, Smad2 and Smad4 mainly expressed in the alveolar type II cell. TβR1 mostly expressed in the membrane; while a tiny portion of its expression can be seen in the cytoplasm. Smad2 and Smad4 mostly expressed in the cytoplasm as well as in the nucleus. In the contrary, the normal lung tissue of the control group showed less obvious staining than the bulla tissues (Figure 3). These results indicated that the expression of TβR1, smd2 and Smad4 of the study group were higher than that in the control group. The IRS score of Smad3 of the study group was higher than that of the control group, but the difference was not statistically significant (P=0.140) (Table 4). But observation through microscope showed that the coloration intensity and density of Smad3 in the bullae tissue was obviously higher than that in the normal tissues of the control group (Figure 3).
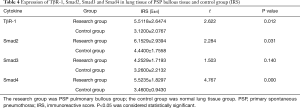
Full table
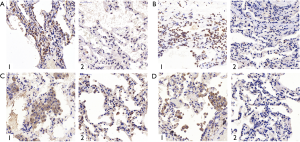
Those results suggested that TβR1, Smad2 and Smad4 highly expressed in the bullae tissues of PSP patients.
The association of expression levels of TβR1, Smad2 and Smad4 and the clinical characteristics of PSP patients
The expression of TβR1, Smad2, Smad3 and Smad4 were not statistically correlated with the clinical characteristics of PSP patients mentioned below, such as age, sex, BMI, recurrence and side involved (P>0.05) (Table 5). It suggested that the expression of cytokines may be not related to these clinical characteristics.

Full table
Discussion
According to our results, TβR1, Smad2 and Smad4 highly expressed in pulmonary bullae tissue tested by immunohistochemistry staining, compared with that in normal lung tissues. Thus we speculated that the high expression of TβR1, Smad2, and Smad4 might play a role in the development of pulmonary bullae.
Transforming growth factor beta superfamily is reported to participate in the mediation of cell regulating, growth, differentiation, apoptosis and interstitial synthesis (12). One of its family members, TGF-β1, which is focused on by many researches, is demonstrated to be involved in the induction, initiation and development of pulmonary fibrosis. Das et al. found that TGF-β1 had a fibrotic effect on either mice or human lung, by in vitro and in vivo experiments (13). In addition, anti-fibrosis drug IFN-gamma-1b can significantly reduce the level of TGF-β1 in lung tissue (14). These results hint that TGF-β1 plays an important role in pulmonary fibrosis. The fibrosis effect of TGF-β1 is depends on the TGF-β/Smads signaling conduction pathway (15).
The development of PSP may be related to pulmonary fibrosis. Some studies suggest that the histopathologic feature of PSP lesion is alveolar collapse accompanied by fibroelastosis. And these pathological findings are not secondary to pneumothorax but have existed before the episode of pneumothorax. We have verified this finding by H&E staining experiments of pulmonary bullae tissue (Figure 1). One hypothesis is that as thickened pleura with fibroelastosis becomes less compliant, and pleural thickening is uneven, areas of thinner apical visceral pleura, which may occasionally be at the site of subpleural bullae, receive a higher negative pressure and thus become likely to rupture (5). During developing of pulmonary fibrosis, the epithelial-mesenchymal transition (EMT) of fibroblasts is an important mechanism through which fibrous cells are evolved. In alveolar epithelial cells, activated TGF-β mediates EMT by activating the Smad pathway and the non-Smad pathway. At the same time, some studies have shown that inhibitors of Smads pathway can prevent the pulmonary from fibrosis by block the TGF-β/Smads pathway (15,16).
It can be seen that the main components of the TGF-β/Smads signal pathway play an important role in the progression of pulmonary fibrosis. Our results showed that TβR1, Smad2 and Smad4 in the pathways highly expressed in pulmonary bullae tissue, suggesting that the development of PSP bullae may be related to pulmonary fibrosis.
Furthermore, when the immunohistochemistry staining results of bullae tissue of 34 PSP patients were compared to the normal lung tissues of control group, it shows that the expression of TβR1, Smad2 and Smad4 were significantly higher than that of control group (P<0.05). The expression level of Smad3 was not statistically difference between the study group and the control group, but the absolute value of the expression in the PSP bullae tissue was higher than that in the normal control group. These results suggested that TβR1, Smad2, Smad4 and Smad3 may be related to the development of PSP bullae.
It is thought that TGF-β and its downstream cytokines which highly express in PSP bullae might induce pulmonary fibrosis. TβR is a receptor protein that can bond tightly with TGF-β on the surface of cell membrane. TβR1 and TβR2 are the main receptors involved in TGF-β signal transduction. After activated, TGF-β must be combined with TβR to form the transmembrane complex which can induce fibrosis. As its downstream proteins, Smads family then conducts signaling from the membrane surface into the nucleus. The activated TGF-β firstly binds to TβR1 and TβR2, forming a complex which in turn activates the downstream Smad pathway. After the activation of Smad families, activated Smad2, Smad3 and Smad4 can enter the nucleus and regulate transcription of downstream genes. Smad4 is a core transduction of the TGF-β signaling pathway, and only with the presence of Smad4 can the TGF-β signal be transferred into the nucleus to regulate transcription (17-19). It indicates that TβR1, Smad2, Smad3 and Smad4 play an important role in TGF-β signaling pathway.
In this study, we found that TβR1, Smad2 and Smad4 highly expressed in PSP bullae. Because TβR1, Smad2 and Smad4 are important factors in TGF-β signaling pathway which mediates pulmonary fibrosis, it hints that the development of PSP may be related to pulmonary fibrosis.
In summary, the results of our study suggested that TβR1, Smad2 and Smad4 might play a role in the development of PSP bullae. Yet, the pathogenesis of pulmonary bullae formation in patients with spontaneous pneumothorax is not yet clearly understood, and the associated hypotheses need further study to be confirmed.
Acknowledgements
Funding: This work was supported by the Basic and Clinical Cooperation Research Fund of China Capital Medical University (15JL39).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Research Ethics Committee of Beijing Chaoyang Hospital, Capital Medical University (ID: 2016-S-121). All the patients signed the informed consent preoperatively. The study outcomes will not affect the future management of the patients.
References
- Bense L, Eklund G, Wiman LG. Smoking and the increased risk of contracting spontaneous pneumothorax. Chest 1987;92:1009-12. [Crossref] [PubMed]
- Melton LJ 3rd, Hepper NG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis 1979;120:1379-82. [PubMed]
- Lichter I, Gwynne JF. Spontaneous pneumothorax in young subjects. A clinical and pathological study. Thorax 1971;26:409-17. [Crossref] [PubMed]
- Belchis DA, Shekitka K, Gocke CD. A unique, histopathologic lesion in a subset of patients with spontaneous pneumothorax. Arch Pathol Lab Med 2012;136:1522-7. [Crossref] [PubMed]
- Kobayashi NS, Nambu A, Kawamoto M, et al. Pulmonary Apical Opacities on Thin-Section Computed Tomography: Relationship to Primary Spontaneous Pneumothorax in Young Male Patients and Corresponding Histopathologic Findings. J Comput Assist Tomogr 2018;42:33-8. [PubMed]
- Zheng X, Qi C, Zhang S, et al. TGF-beta1 induces Fstl1 via the Smad3-c-Jun pathway in lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 2017;313:L240-51. [Crossref] [PubMed]
- Sibinska Z, Tian X, Korfei M, et al. Amplified canonical transforming growth factor-beta signalling via heat shock protein 90 in pulmonary fibrosis. Eur Respir J 2017.49. [PubMed]
- Tomcik M, Zerr P, Pitkowski J, et al. Heat shock protein 90 (Hsp90) inhibition targets canonical TGF-beta signalling to prevent fibrosis. Ann Rheum Dis 2014;73:1215-22. [Crossref] [PubMed]
- Prabowo AS, Iyer AM, Veersema TJ, et al. Expression of neurodegenerative disease-related proteins and caspase-3 in glioneuronal tumours. Neuropathol Appl Neurobiol 2015;41:e1-15. [Crossref] [PubMed]
- Gatto F, Feelders RA, van der Pas R, et al. Immunoreactivity score using an anti-sst2A receptor monoclonal antibody strongly predicts the biochemical response to adjuvant treatment with somatostatin analogs in acromegaly. J Clin Endocrinol Metab 2013;98:E66-71. [Crossref] [PubMed]
- Liu LK, Jiang XY, Zhou XX, et al. Upregulation of vimentin and aberrant expression of E-cadherin/beta-catenin complex in oral squamous cell carcinomas: correlation with the clinicopathological features and patient outcome. Mod Pathol 2010;23:213-24. [Crossref] [PubMed]
- Thomas BJ, Kan OK, Loveland KL, et al. In the Shadow of Fibrosis: Innate Immune Suppression Mediated by Transforming Growth Factor-beta. Am J Respir Cell Mol Biol 2016;55:759-66. [Crossref] [PubMed]
- Das S, Kumar M, Negi V, et al. MicroRNA-326 regulates profibrotic functions of transforming growth factor-beta in pulmonary fibrosis. Am J Respir Cell Mol Biol 2014;50:882-92. [Crossref] [PubMed]
- Bouros D, Antoniou KM, Tzouvelekis A, et al. Interferon-gamma 1b for the treatment of idiopathic pulmonary fibrosis. Expert Opin Biol Ther 2006;6:1051-60. [Crossref] [PubMed]
- Kolosova I, Nethery D, Kern JA. Role of Smad2/3 and p38 MAP kinase in TGF-beta1-induced epithelial-mesenchymal transition of pulmonary epithelial cells. J Cell Physiol 2011;226:1248-54. [Crossref] [PubMed]
- Derynck R, Muthusamy BP, Saeteurn KY. Signaling pathway cooperation in TGF-beta-induced epithelial-mesenchymal transition. Curr Opin Cell Biol 2014;31:56-66. [Crossref] [PubMed]
- Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997;390:465-71. [Crossref] [PubMed]
- Macias MJ, Martin-Malpartida P, Massague J. Structural determinants of Smad function in TGF-beta signaling. Trends Biochem Sci 2015;40:296-308. [Crossref] [PubMed]
- Ok Atılgan A, Ozdemir BH, Akcay EY, et al. Role of tumor-associated macrophages in the Hexim1 and TGFbeta/SMAD pathway, and their influence on progression of prostatic adenocarcinoma. Pathol Res Pract 2016;212:83-92. [Crossref] [PubMed]


