Adjuvant EGFR TKI therapy for resectable non-small cell lung cancer: new era for personalized medicine
Lung cancer is now the leading cause of cancer death in both men and women in China, accounting for 21.7% of estimated cancer deaths in 2015 (1). Lung cancer is also the leading cause of cancer death in men and the second leading cause of cancer death in women worldwide (2). Non-small cell lung cancer (NSCLC) comprise 85% of all lung cancers and surgery is the recommended treatment for patients with stage I–II NSCLC (3,4). Despite about 20–25% of patients diagnosed with NSCLC are suitable for surgery management (5), the 5-year survival rate of resected NSCLC ranges from 25% to 73% according to pathological stage (6). The recommended adjuvant treatment for stage IIA–IIIB resected NSCLC-regardless of EGFR mutation status is cisplatin-doublet chemotherapy which the most frequently studied regimen is cisplatin-vinorelbine (4,7-9), which has shown increased overall survival and disease-free survival compared with that for observation (10).
The Lung Adjuvant Cisplatin Evaluation (LACE) meta-analysis pooled 4,584 patients in five large cisplatin-based adjuvant trials and confirmed the benefit of adjuvant chemotherapy, with a 5.3% and 5.2% improvement of survival and DFS at 5 years (P=0.0043, P<0.0001). For different pathological stages, its value in lower stages is less clear. Postoperative adjuvant chemotherapy showed a negative effect in stage IA. The risk reduction was 8% for stage IB and 17% for stages II and III (11). A subgroup analysis indicated it was mainly due to the outcome in patients with tumors ≥4 cm (12,13). However, regardless of whether adjuvant chemotherapy has been administered or not, the 5-year survival of these patients remains poor. Meanwhile, the recently reported E1505 study, a phase III randomized trial of adjuvant chemotherapy with or without bevacizumab in patients with resected NSCLC [stage IB (≥4 cm) to IIIA], showed the addition of bevacizumab did not improve overall survival and indicated this combination should not be considered as an adjuvant therapy for patients in this setting (14).
Epidermal growth factor receptor (EGFR) activating mutations, which occur in approximately 51.4% of advanced NSCLC patients with adenocarcinoma in Asia (15), 10–17% in European and United States patients (16,17), correlated with tumor response to EGFR tyrosine kinase inhibitors (TKIs). The best described activating EGFR alterations are mutations in exon 19 (ex19del) and exon 21 (L858R), which account for approximately 90% of all EGFR-mutated NSCLCs (17). Worldwide phase III clinical trials involving advanced NSCLC patients with activating EGFR mutations have demonstrated high response rates (~70%) and significantly longer progression-free survival (PFS) in patients treated with EGFR TKIs (gefitinib, erlotinib, afatinib, osimertinib) as first-line treatment when compared with those receiving doublet chemotherapy (18-23). The remarkable efficacy of EGFR TKIs in this unique subset of patients has revolutionized the therapeutic approach to lung cancer that making EGFR TKIs as standard first-line treatment for EGFR-mutant advanced NSCLC nowadays.
Whether the success of EGFR TKIs in the advanced setting could transfer to adjuvant setting for patients with early-stage disease attracts much more attention. The use of EGFR TKI adjuvant therapy for EGFR-mutated non-metastatic lung cancer remains controversial because there is less conclusive evidence to support its use, even a few prospective studies have been published.
BR19, a phase III randomized controlled double blinded trial, performed adjuvant gefitinib in unselected patients with stage IB–IIIA resected NSCLC treat or not with adjuvant platinum-based chemotherapy. Unfortunately, the study was prematurely closed after randomizing only 503 of the 1,242 patients that were initially planned and the median duration of gefitinib therapy was 4.8 months. Only fifteen patients had activating EGFR mutations of whom seven received gefitinib and eight received placebo (24). This was an underpowered study due to lack relevant patient population and short duration of treatment.
Another randomized phase 3 trial, RADIANT, was a placebo-controlled trial of adjuvant erlotinib versus placebo for patients with completely resected stages IB–IIIA (25). Patients with EGFR positivity confirmed by immunohistochemistry and/or fluorescence in situ hybridization were randomized (2:1) to either erlotinib or placebo for 2 years. The primary endpoint was disease-free survival (DFS). The study showed there was no significant difference in DFS between erlotinib and placebo groups [50.5 vs. 48.2 months, hazard ratio (HR) =0.90; 95% CI: 0.74–1.10; P=0.324], while median overall survival data were immature with a median follow-up of 47 months. Notably, the median duration of erlotinib treatment was shorter than placebo (11.9 vs. 21.9 months). Interestingly, subgroup analysis of patients with EGFR mutation (ex19del and 21 L858R) represented a trend in favor of erlotinib (46.4 vs. 28.5 months, HR =0.61; 95% CI: 0.38–0.98, P=0.04).
Recent studies which either uncontrolled studies (26) or randomized phase II studies (19) focused exclusively on EGFR mutation patients, showed promising results in terms of clinical outcome. Unfortunately, they were underpowered to lead to definitive results that could change clinical practice.
ADJUVANT/CTONG1104, an international randomized, double-blind, the first head-to-head, prospective, phase 3 trial, compared adjuvant gefitinib with vinorelbine plus cisplatin in patients with resected stage II–IIIA (N1–N2) NSCLC harboring EGFR mutations (ex19del or 21 L858R) (27). Four hundred and eighty-three patients were screened for study inclusion and 222 patients with EGFR-mutant NSCLC were randomly allocated (1:1) to either gefitinib group or vinorelbine plus cisplatin group. The regimens were either oral gefitinib 250 mg once per day for 2 years or intravenous vinorelbine 25 mg/m2 (day 1,8) plus cisplatin 75 mg/m2 (day 1) every 3 weeks for four cycles (12 weeks in total). The primary endpoint was DFS in the intention-to-treat (ITT) population, which comprised all randomised patients. The secondary endpoints were overall survival; 3-year DFS, 5-year DFS, and 5-year overall survival (OS); safety and tolerability; and health-related quality of life (HRQoL). Of note, median DFS was 10.7 months significantly longer in gefitinib group than vinorelbine plus cisplatin group (28.7 vs. 18.0 months, HR =0.60, P=0.0054). Three-year DFS in the ITT population was 34% and 27% in the two groups respectively (HR =0.74, P=0.37). DFS analysis in the preset subgroups showed gefitinib provided a consistent benefit compared with that for chemotherapy. In term of safety and tolerability, the most commonly reported grade 3 or worse adverse events (AEs) in two groups were consistent with the profile reported previously (18,19,28). The frequency of overall AEs reported in gefitinib was reduced compared with that of vinorelbine plus cisplatin, the same reduction was found in the grade 3 or worse AEs. Furthermore, there were 12 (11%) patients needed dose adjustment in gefitinib compared with twenty-nine (33%) patients in vinorelbine plus cisplatin. Discontinued treatment was reported by three (3%) patients who received gefitinib and 5 (6%) who received vinorelbine plus cisplatin due to drug-related toxicity. Trial Outcome Index (TOI) scores from baseline to week 33 were significantly improved in patients who received gefitinib compared with those who received vinorelbine plus cisplatin (P=0.012).
Importantly, there are three points deserved to be mentioned. First of all, the patients were recruited only stage II–IIIA with N1 or N2 disease who are at higher risk of disease recurrence and benefit from adjuvant chemotherapy, differed from that in the BR19 (24) and RADIANT (25) studies (stage IB–IIIA NSCLC) (11). Also, the EGFR-activating mutations were ascertained using amplification-refractory mutation system PCR, not IHC or FISH (25). Second, the median treatment duration of EGFR-TKIs was 21.9 months in ADJUVANT study, which was comparable with that reported in the SELECT study (20.0 months) (26) and longer than those reported in the BR19 study (4.8 months) (24) and RADIANT study (11.9 months) (25). Third, EGFR-TKI was administered first, head to head compared with chemotherapy in ADJUVANT trial. However, in the SWOG S0023 study, gefitinib or placebo were administered after concurrent chemoradiotherapy and docetaxel consolidation, as maintenance treatment for inoperable stage III NSCLC (29). In the BR19 (24) and RADIANT (25) studies, EGFR TKIs were compared with placebo in the adjuvant setting and administered after previous treatment with chemotherapy.
Overall, ADJUVANT study shows that gefitinib treatment indicated significantly longer DFS than that of vinorelbine plus cisplatin in patients with completely resected (R0), stage II–IIIA (N1–N2), EGFR-mutant NSCLC. Though, the overall survival data were immature, with a median follow up of 36.5 months at the time of publication. We could speculate that some patients will still benefit from adjuvant gefitinib treatment on account of increased DFS, reduced toxicity, and higher quality of life.
However, there are also important issues regarding adjuvant EGFR TKIs treatment.
Which patients need adjuvant targeted therapy?
The rationale for adjuvant treatment is its potential to eradicate minimal residual disease (MRD). So, one critical issue is to select favored patients when initiate adjuvant therapy, because the treatment is essentially for a risk, rather than for provable disease. Obviously, TNM stage is a common criteria. It seems higher TNM stage benefit more from EGFR TKI adjuvant therapy. A prospective, open-labelled, randomized, multicenter phase 2 “EVAN” study (NCT01683175) evaluate efficacy and safety of erlotinib vs. Cis-platinum/Vinorelbine chemotherapy as adjuvant therapy in stage IIIA NSCLC patients with EGFR mutation. This study reported 2-year disease free survival rate (DFSR) was significantly higher in erlotinib group than chemotherapy group (81.35% vs. 44.62%, P<0.001). Three-year DFSR remained the same trend. Median DFS was 21.45 months significantly longer in erlotinib group than chemotherapy group (42.41 vs. 20.96 months, HR =0.268, P<0.001). Besides TNM stage, molecular status may provide more information for patient’s selection. In most ongoing trials, the patient’s inclusion was based on EGFR ex19del and 21L858R. However, conjoint analysis of LUX-lung 3 and LUX-lung 6 trials showed that patients with EGFR ex19del has significantly longer overall survival than patients with EGFR 21L858R (33.3 vs. 27.6 months in LUX-lung 3, 31.4 vs. 19.6 months in LUX-lung 6) (30). How EGFR mutation status affect the adjuvant EGFR TKIs treatment need further research. Liquid biopsy represents a promising strategy to help identify the high-risk population or perform disease surveillance via measurement of circulating tumor cells (CTCs), circulating tumor DNA (ctDNA) or other biomarkers.
Which generation of EGFR TKIs is the best choice to use?
In the majority of published adjuvant EGFR TKIs studies, the first-generation EGFR TKIs (gefitinib, erlotinib, icotinib) were performed. However, the third-generation EGFR TKI osimertinib has showed efficacy superior to that of gefitinib or erlotinib in first-line treatment of EGFR-mutated advanced NSCLC (31). With regard to this, the use of adjuvant osimertinib might provide benefits, and the ongoing international phase 3 study “ADAURA” compare osimertinib versus placebo in EGFR-mutated NSCLC who have had complete tumor resection, with or without postoperative adjuvant chemotherapy (NCT02511106).
What is the optimal treatment duration of adjuvant EGFR-TKI?
Actually, in most clinical trials, adjuvant EGFR TKIs therapy continued for 2 years, in the absence of disease relapse, death, or unacceptable toxic effects. In the RADIANT study, the median duration of erlotinib treatment was only 12 versus 22 months for placebo, which erlotinib treatment was arranged to be given for 2 years. In addition, the incidence of overall AEs in erlotinib group was significantly higher than that of placebo group. AEs related dose reduction, interruption, or both was 22.0%, 22.0%, and 34.0% in erlotinib arm versus 1.7%, 6.8%, and 1.7% in placebo arm (25). But then, it is possible that prolonged EGFR TKIs exposure will result in greater benefits. A phase II trial are ongoing to see which works better either 3 months (short course) compared to 2 years (long course) afatinib after surgery in NSCLC patients with EGFR mutation (NCT01746251). Another ongoing phase III Trial “ICTAN” attempt to answer how well it works that adjuvant icotinib for 6- or 12-month following chemotherapy compared with chemotherapy in treating EGFR mutation patients with resected stage IIA–IIIA NSCLC (NCT01996098). Anyway, duration should balance the side effects with the benefits of treatment.
Which adjuvant regimen is best for EGFR mutation patients?
In previous published and ongoing randomized clinical trials, EGFR TKIs is mostly used stand-alone versus placebo or chemotherapy to answer whether it’s superior to the latter treatment. Meanwhile, there are studies which EGFR TKIs was administrated sequentially after platinum-based chemotherapy. A phase 2 study, in patients with resected stage IIIA-N2 NSCLC harbouring EGFR mutations, reported DFS was significantly longer among those who received pemetrexed and carboplatin (PC) followed with gefitinib than among those who received PC alone (39.8 vs. 27.0 months, P=0.014). The rates of 2-year overall survival were 92.4% and 77.4% in two groups respectively (P=0.076) (32). Also, erlotinib was administrated following chemotherapy in the ongoing ALCHEMIST trial, the same administration of icotinib in the ongoing ICTAN trial. JMIT study has showed significantly longer PFS in pemetrexed plus gefitinib than gefitinib monotherapy (median, 15.8 vs. 10.9 months, P=0.014) in patients with advanced nonsquamous NSCLC and EGFR mutations (33). Could this consolidation therapy prove beneficial in adjuvant setting? Another randomized phase III trial is studying gefitinib and synchronous pemetrexed plus cisplatin chemotherapy compared to pemetrexed plus cisplatin chemotherapy alone in adjuvant treating stage II–IIIA (N1-N2) lung adenocarcinoma patients with EGFR activating mutation in Asian population (NCT02518802). Is there another combined treatment could be better? In stage IIIA-N2 NSCLC, there also is one phase II trial to explore the survival benefit of the gefitinib combined with radiotherapy as adjuvant therapy for completely resected patients harbouring sensitive mutations of EGFR (NCT03381430).
Similar with ADJUVANT study, IMPACT(WJOG6401L), EVIDENCE (NCT02448797) are designed to evaluate the efficacy of gefitinib or icotinib versus vinorelbine/platinum as adjuvant therapy in treating stage II–IIIA NSCLC patients with EGFR mutation. While ICWIP (NCT02125240) trial compare 3-year of DFS of Icotinib and placebo in the treatment of patients who EGFR mutation-positive stage II–IIIA only in lung adenocarcinoma. ALCHEMIST (NCT02193282) has been initiated to investigate the role of adjuvant erlotinib, crizotinib, and nivolumab based on molecular alterations. Specifically, patients with EGFR and ALK wild-type tumors are randomized to nivolumab for up to one year following standard of care.
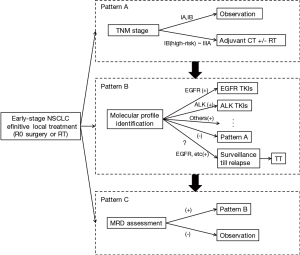
Although adjuvant EGFR TKI debate will continue, we are optimistic that data from the ongoing trials could help to identify if EGFR TKIs do provide an overall survival benefit in the adjuvant setting. Meanwhile, these studies will provide a broad overview of the genomic landscape of NSCLC in early stage, also help us perform better personalized therapy and promote the evolution of adjuvant treatment strategy (Figure 1).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Kerr KM, Bubendorf L, Edelman MJ, et al. Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol 2014;25:1681-90. [Crossref] [PubMed]
- NSCLC Meta-analyses Collaborative Group, Arriagada R, Auperin A, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267-77. [Crossref] [PubMed]
- Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review 1975-2013. Bethesda, MD: National Cancer Institute, 2016.
- Zhi XY, Yu JM, Shi YK. Chinese guidelines on the diagnosis and treatment of primary lung cancer (2015 version). Cancer 2015;121 Suppl 17:3165-81. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. NCCN guidelines insights: non-small cell lung cancer, Version 4.2016. J Natl Compr Canc Netw 2016;14:255-64. [Crossref] [PubMed]
- Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol 2017;35:2960-74. [Crossref] [PubMed]
- Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev 2015.CD011430. [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Strauss GM, Herndon JE, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [Crossref] [PubMed]
- Salazar MC, Rosen JE, Wang Z, et al. Association of delayed adjuvant chemotherapy with survival after lung cancer surgery. JAMA Oncol 2017;3:610-9. [Crossref] [PubMed]
- Wakelee HA, Dahlberg SE, Keller SM, et al. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 2017;18:1610-23. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in asian patients with advanced non–small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- Marchetti A, Martella C, Felicioni L, et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol 2005;23:857-65. [Crossref] [PubMed]
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR Mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320-6. [Crossref] [PubMed]
- Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): a randomized, double-blind, phase III Trial. J Clin Oncol 2015;33:4007-14. [Crossref] [PubMed]
- Pennell NA, Neal JW, Chaft JE, et al. SELECT: A multicenter phase II trial of adjuvant erlotinib in resected early-stage EGFR mutation-positive NSCLC. J Clin Oncol 2014;32:7514.
- Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N1–N2) EGFR -mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018;19:139-48. [Crossref] [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. [Crossref] [PubMed]
- Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 2008;26:2450-6. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Li N, Ou W, Ye X, et al. Pemetrexed-carboplatin adjuvant chemotherapy with or without gefitinib in resected stage IIIA-N2 non-small cell lung cancer harbouring EGFR mutations: a randomized, phase II study. Ann Surg Oncol 2014;21:2091-6. [Crossref] [PubMed]
- Cheng Y, Murakami H, Yang PC, et al. Randomized Phase II trial of gefitinib with and without pemetrexed as first-line therapy in patients with advanced nonsquamous non-small-cell lung cancer with activating epidermal growth factor receptor mutations. J Clin Oncol 2016;34:3258-66. [Crossref] [PubMed]


