Electrical disorders in atrial septal defect: genetics and heritability
Introduction
Atrial septal defect (ASD) is one of the most common types of congenital heart disease (CHD), constituting 8–10% of CHD cases in children. ASD is sometimes associated with complex CHDs or chromosomal abnormality. Although most ASDs occur sporadically, hereditary modes of inheritance have been reported. The risk of CHD in offspring of a woman with sporadic ASD has been reported to be 8–10%(1). Hereditary ASD is sometimes complicated with bradyarrhythmias such as atrioventricular block (AVB) or sick sinus syndrome (SSS); patients with surgically-repaired ASD sometimes present tachyarrhythmias such as incisional atrial tachycardia caused by operation. We herein describe the inherited ASDs associated with arrhythmias: Holt-Oram syndrome (HOS) and ASD with atrioventricular (AV) disturbance. Atrial tachycardia after ASD operational repair will be discussed in a later.
Development of atrial septation and cardiac conduction system
Atrial septation
The atrial septum is formed by three distinct structures: (I) the AV septal cushion; (II) the primary atrial septum; and (III) the dorsal mesenchymal protrusion associated with the second heart field (SHF) (2,3). Transcription factors important during the atrial septation include GATA4, NKzX2-5, and TBX5. TBX5 interacts with NKX2-5 and GATA4 and positively regulates transcription within the developing heart. TBX5 regulates SHF GGAS1 and OSR1, which are required in the posterior SHF for atrial septation (2,4,5).
Cardiac conduction system (Figure 1)
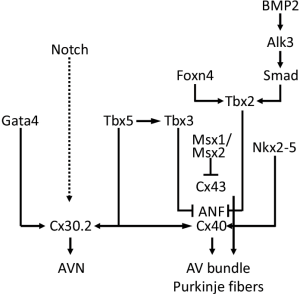
Two transcriptional repressors, TBX2 and TBX3, are crucial in suppressing the further differentiation of the AV canal myocardium (7,8). The combined action of widely expressed transcription factors: NKX2-5, TBX5, and ID2 (inhibitor of DNA binding), the latter having a more limited domain of expression, is now known to be crucial for the development of the AV conduction system (9). TBX3 represses the chamber-specific genes ANF, GJA5, and GJA1 along with TBX2, MSX1, and MSX2. While TBX5 and NKX2-5 activate the expression of ANF and GJA5, TBX5 also activate Cx30.2 expression together with GATA4 (6,10) (Figure 1).
HOS
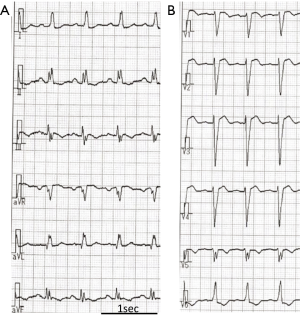
This syndrome, characterized by thumb anomaly and ASD, was first described by Holt and Oram in 1960 (11). They reported a family in which members of four generations were affected by both CHD and skeletal anomalies, and their malformations appeared to be of similar type in all the affected members: ASD, often accompanied by bizarre arrhythmias, and the skeletal anomalies affected mainly the hands. Their electrocardiograms (Figure 2) showed sinus bradycardia, PR prolongation, and escape junctional beats. One family member had sudden cardiac death (SCD). The inheritance followed Mendelian dominant type.
The combination (ASD + finger anomaly) is the most common type of “heart-hand” syndrome (upper limb and CHDs) and is estimated to occur in 1 of 100,000 live births (12). Linkage analysis performed on families demonstrating autosomal dominant inheritance identified a disease locus at 12q24 in 1994 (13). Subsequent investigations identified TBX5 mutations in patients with HOS at 1997 (14,15). TBX5 is a member of a family of transcription factor genes characterized by a highly conserved binding domain called T-box (16). It is located on 12q24.1 and consists of 10 exons.
Clinical features: a congenital heart malformation is present in 75% of clinically diagnosed patients with HOS (10). Although atrial and ventricular septal defects are most commonly seen, respectively occurring in 58% and 28% of patients, additional CHDs have also been reported, including AV septal defects, tetralogy of Fallot, truncus arteriosus, partial pulmonary venous drainage, ductus arteriosus, or hypoplastic left heart syndrome (17,18). Cardiac involvement was found in 95% of familial cases. Simple CHD was found in 66% of patients and more complex CHD in 17.5%. Some cases showed only ECG abnormalities without CHD (18).
Cardiac conduction diseases include bradycardia, AVB, atrial fibrillation, sinus node dysfunction, or right bundle branch block (13). Tbx5 knock-out mice display a maturation failure of the conduction system morphology and function. In the AV canal of the heterozygous Tbx5del/+ mouse heart, cells failed to mature into discrete AV node or bundle. In clinically severe cases, the discrete right bundle branch is completely absent on the right ventricular septal surface. ECG of Tbx5del/+ mice shows the prolongation of the PR and QRS intervals, and a broad P wave. Sinus pause is frequent and prolonged with 24 hours monitoring (19,20).
Skeletal anomalies involve the preaxial radial ray and are fully penetrant. Subclinical changes may include only radiographic evidence of abnormal carpal bone, whereas others have obvious severe manifestations such as phocomelia (13,18). Upper-limb malformations include unequal arm length, fusion or anomalous development of the carpal and thenar bones, abnormal forearm pronation and supination, abnormal opposition of the thumb, and sloping shoulders and restriction of shoulder joint movement (11). Carpal abnormalities are more specific for the HOS than are changes in the thumb (21). The left side is reported to be more severely affected (22). Other skeletal anomalies were polydactyly, arachnodactyly, coxa vara, thoracic scoliosis, hemi-atrophy of the body, and hypoplastic mandible.
Other congenital malformations found in HOS are miscellaneous: lung hypoplasia, malformations of renal and cerebral arteries, hypoplastic peripheral upper extremity vasculature, hypoplasia of left radial artery, pulmonary hypertension, cardiomyopathy, Hirschsprung disease, malformation of urinary system, renal failure, cryptorchidism, the Rokitansky-Kuster-Hauser syndrome (immature or absent of vagina or uterus), multiple strokes, myopia, and malignant tumors (23).
The correlation of the genotype with the severity of clinical features: TBX5 mutations are identified in 70% of patients with the clinical features of HOS (24,25). The genotype-phenotype correlation in HOS remains controversial. More than 90 mutations have been identified within the TBX5 gene, including missense, nonsense, frameshift mutations, splice mutations, duplication, and chromosomal rearrangements (14,15,26-31). Most mutations were predicted to encode a truncated protein and cause haploinsufficiency leading to more severe limb and cardiac phenotype. Of 90 these mutations, missense mutations (n>50) cause a large phenotype variation depending on their position within the DNA-binding domain. TBX5 dosage would be crucial for the healthy development of heart and upper limb (26).
A missense mutation (p.Pro85Thr) was found to result in a switch from cis-peptide conformation to the trans-conformation bond, affecting both folding and biological function and thereby leading to loss of function (31). Amino acid alteration near the amino-terminal end of the T-box, which should affect interactions with the major groove of the target DNA sequence, produces very significant cardiac malformations and minor skeletal abnormalities. In contrast, change in amino acids at the carboxyl end of the T-box, which should affect interactions between TBX5 and the minor groove of the DNA target sequence, produces severe limb abnormalities and less significant cardiac abnormalities (27). It has also been reported, however, that neither the type of mutation in TBX5 nor the location of a mutation in the T-box was predictive of the expression of malformations (32). Two gain-of-function mutations were associated with HOS (33,34).
ASD with AV conduction defects associated with NKX2-5 gene mutation
Hereditary ASD with prolonged PR interval, AVB and juvenile SCD has been reported in association with NKX2-5 mutations (35-38). While the cardiac manifestations can be similar to those found in HOS, the disease associated with NKX2-5 mutation lacks extracardiac anomalies such as upper limb malformations.
Schott et al. first demonstrated NKX2-5 mutations in four families harboring AVB and CHD, including ASD or tetralogy of Fallot (39). Their 33 affected individuals all presented AV conduction delays, for which 14 received pacemaker implantation. SCD occurred in six asymptomatic individuals without pacemakers after surgical ASD repair. NKX2-5 mutations also have been identified in patients with dilated cardiomyopathy (40,41) or ventricular arrhythmias (42) and caused SCD (43). SCD occurred in 15% of the patients with hereditary ASD and AV conduction disturbance (44). The families with ASD and SCD have NKX2-5 mutations in 44% (44). The autopsy study revealed the fibrotic replacement of the AV bundle (45). Intra- and inter-familial phenotype variability of NKX2-5 mutations is noted, but so far, no correlation has been reported between the mutant alleles and the severity in phenotype (46). In cases of hereditary ASD or CHD, or ASD with AV conduction disturbances, the genetic testing of NKX2-5 would be useful for predicting ventricular arrhythmias or SCD. If they are mutation carriers, a preventable implantable cardioverter defibrillator would be indicated in these patients (42,44).
NKX2-5 gene mutation: the NKX2-5 gene is located on chromosome 5 (5q34), which contains two exons encoding a 324-amino-acid protein. As a transcription factors of the nucleotide kinase (NK)-2 class, NKX2-5 contains three highly conserved regions: the homeodomain, the tinman domain and the NK2 domain (47). Nkx2-5 is necessary for cardiac development as well as maintaining proper function of the AV node and myocardium throughout adult life (48).
Homozygous inactivation of Nkx2-5 in mammals impairs cardiac looping and is embryonic lethal (49,50). Mice with a heterozygous cardiac-specific Nkx2-5 deletion exhibited a more subtle phenotype, which consisted of ASD, abnormality of aortic valve, and, in female mice, prolongation of the PR interval (51). To date, 61 different mutations have been identified (39,41,44,52-57). Schott et al. (39) also reported three different NKX2-5 mutations (Gln170Ter, Thr178Met, and Gln198Ter) in patients with ASD and AV conduction disturbance. Two mutations were located in the DNA-binding homeodomain of NKX2.5 and are likely to alter the affinity of target DNA binding, the other two were found to result in the deletion of C-terminal amino acids aberrantly augmenting transcription of downstream genes. Most of the NKX2-5 mutations (62%) are amino-acid substitutions. The rest are premature stop codons (46).
Summary
Recent extensive molecular study on transcriptional factors has elucidated the genetic background of hereditary ASD accompanied with arrhythmias. We need to carefully examine not only cardiac problems, but also the whole body of patients with ASD. Especially in the case of hereditary ASD, genetic testing will prevent future unexpected SCD due to bradycardia or tachycardia in both probands and their family members.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siu SC, Colman JM, Sorensen S, et al. Adverse neonatal and cardiac outcomes are more common in pregnant women with cardiac disease. Circulation 2002;105:2179-84. [Crossref] [PubMed]
- Xie L, Hoffmann AD, Burnicka-Turek O, et al. Tbx5-hedgehog molecular networks are essential in the second heart field for atrial septation. Dev Cell 2012;23:280-91. [Crossref] [PubMed]
- Galli D, Dominguez JN, Zaffran S, et al. Atrial myocardium derives from the posterior region of the second heart field, which acquires left-right identity as Pitx2c is expressed. Development 2008;135:1157-67. [Crossref] [PubMed]
- Zhou L, Liu J, Olson P, et al. Tbx5 and Osr1 interact to regulate posterior second heart field cell cycle progression for cardiac septation. J Mol Cell Cardiol 2015;85:1-12. [Crossref] [PubMed]
- Zhang KK, Xiang M, Zhou L, et al. Gene network and familial analyses uncover a gene network involving Tbx5/Osr1/Pcsk6 interaction in the second heart field for atrial septation. Hum Mol Genet 2016;25:1140-51. [Crossref] [PubMed]
- Munshi NV. Gene regulatory networks in cardiac conduction system development. Circ Res 2012;110:1525-37. [Crossref] [PubMed]
- Christoffels VM, Hoogaars WM, Tessari A, et al. T-box transcription factor Tbx2 represses differentiation and formation of the cardiac chambers. Dev Dyn 2004;229:763-70. [Crossref] [PubMed]
- Harrelson Z, Kelly RG, Goldin SN, et al. Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the outflow tract during heart development. Development 2004;131:5041-52. [Crossref] [PubMed]
- Moskowitz IP, Kim JB, Moore ML, et al. A molecular pathway including Id2, Tbx5, and Nkx2-5 required for cardiac conduction system development. Cell 2007;129:1365-76. [Crossref] [PubMed]
- Allen HD, Driscoll DJ, Shaddy RE, et al. Moss and Adams' Heart Disease in Infant, Children, and Adolescents. 9th edition ed. Wolters Kluwer; 2016.
- Holt M, Oram S. Familial heart disease with skeletal malformations. Br Heart J 1960;22:236-42. [Crossref] [PubMed]
- Elek C, Vitez M, Czeizel E. Holt-Oram syndrome. Orv Hetil 1991;132:73-4, 77-8. [PubMed]
- Basson CT, Cowley GS, Solomon SD, et al. The clinical and genetic spectrum of the Holt-Oram syndrome (heart-hand syndrome). N Engl J Med 1994;330:885-91. [Crossref] [PubMed]
- Basson CT, Bachinsky DR, Lin RC, et al. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet 1997;15:30-5. [Crossref] [PubMed]
- Li QY, Newbury-Ecob RA, Terrett JA, et al. Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat Genet 1997;15:21-9. [Crossref] [PubMed]
- Packham EA, Brook JD. T-box genes in human disorders. Hum Mol Genet 2003;12:R37-44. [Crossref] [PubMed]
- Bruneau BG, Logan M, Davis N, et al. Chamber-specific cardiac expression of Tbx5 and heart defects in Holt-Oram syndrome. Dev Biol 1999;211:100-8. [Crossref] [PubMed]
- Newbury-Ecob RA, Leanage R, Raeburn JA, et al. Holt-Oram syndrome: a clinical genetic study. J Med Genet 1996;33:300-7. [Crossref] [PubMed]
- Moskowitz IP, Pizard A, Patel VV, et al. The T-Box transcription factor Tbx5 is required for the patterning and maturation of the murine cardiac conduction system. Development 2004;131:4107-16. [Crossref] [PubMed]
- Bruneau BG, Nemer G, Schmitt JP, et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell 2001;106:709-21. [Crossref] [PubMed]
- Poznanski AK, Gall JC Jr, Stern AM. Skeletal manifestations of the Holt-Oram syndrome. Radiology 1970;94:45-53. [Crossref] [PubMed]
- Smith AT, Sack GH Jr, Taylor GJ. Holt-Oram syndrome. J Pediatr 1979;95:538-43. [Crossref] [PubMed]
- Huang T. Current advances in Holt-Oram syndrome. Curr Opin Pediatr 2002;14:691-5. [Crossref] [PubMed]
- McDermott DA, Bressan MC, He J, et al. TBX5 genetic testing validates strict clinical criteria for Holt-Oram syndrome. Pediatr Res 2005;58:981-6. [Crossref] [PubMed]
- Debeer P, Race V, Gewillig M, et al. Novel TBX5 mutations in patients with Holt-Oram syndrome. Clin Orthop Relat Res 2007.20-6. [Crossref] [PubMed]
- Kimura M, Kikuchi A, Ichinoi N, et al. Novel TBX5 duplication in a Japanese family with Holt-Oram syndrome. Pediatr Cardiol 2015;36:244-7. [Crossref] [PubMed]
- Basson CT, Huang T, Lin RC, et al. Different TBX5 interactions in heart and limb defined by Holt-Oram syndrome mutations. Proc Natl Acad Sci U S A 1999;96:2919-24. [Crossref] [PubMed]
- Yang J, Hu D, Xia J, et al. TBX5 mutation in Chinese patients with Holt-Oram syndrome. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2000;17:233-5. [PubMed]
- Gruenauer-Kloevekorn C, Froster UG. Holt-Oram syndrome: a new mutation in the TBX5 gene in two unrelated families. Ann Genet 2003;46:19-23. [Crossref] [PubMed]
- Al-Qattan MM, Abou Al-Shaar H. A novel missense mutation in the TBX5 gene in a Saudi infant with Holt-Oram syndrome. Saudi Med J 2015;36:980-2. [Crossref] [PubMed]
- Dressen M, Lahm H, Lahm A, et al. A novel de novo TBX5 mutation in a patient with Holt-Oram syndrome leading to a dramatically reduced biological function. Mol Genet Genomic Med 2016;4:557-67. [Crossref] [PubMed]
- Brassington AM, Sung SS, Toydemir RM, et al. Expressivity of Holt-Oram syndrome is not predicted by TBX5 genotype. Am J Hum Genet 2003;73:74-85. [Crossref] [PubMed]
- Postma AV, van de Meerakker JB, Mathijssen IB, et al. A gain-of-function TBX5 mutation is associated with atypical Holt-Oram syndrome and paroxysmal atrial fibrillation. Circ Res 2008;102:1433-42. [Crossref] [PubMed]
- Ye M, Parente F, Li X, et al. Gene-targeted deletion of OPCML and Neurotrimin in mice does not yield congenital heart defects. Am J Med Genet A 2014;164A:966-74. [Crossref] [PubMed]
- Kahler RL BE, Plauth WH Jr. Familial congenital heart disease: Familial occurrence of atrial septal defect with A-V conduction abnormalities; supravalvular aortic and pulmonic stenosis; and ventricular septal defect. Amer J Med 1966.384. [Crossref]
- Bizarro RO, Callahan JA, Feldt RH, et al. Familial atrial septal defect with prolonged atrioventricular conduction. A syndrome showing the autosomal dominant pattern of inheritance. Circulation 1970;41:677-83. [Crossref] [PubMed]
- Emanuel R, O'Brien K, Somerville J, et al. Association of secundum atrial septal defect with abnormalities of atrioventricular conduction or left axis deviation. Genetic study of 10 families. Br Heart J 1975;37:1085-92. [Crossref] [PubMed]
- Pease WE, Nordenberg A, Ladda RL. Familial atrial septal defect with prolonged atrioventricular conduction. Circulation 1976;53:759-62. [Crossref] [PubMed]
- Schott JJ, Benson DW, Basson CT, et al. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science 1998;281:108-11. [Crossref] [PubMed]
- Ouyang P, Saarel E, Bai Y, et al. A de novo mutation in NKX2.5 associated with atrial septal defects, ventricular noncompaction, syncope and sudden death. Clin Chim Acta 2011;412:170-5. [Crossref] [PubMed]
- Costa MW, Guo G, Wolstein O, et al. Functional characterization of a novel mutation in NKX2-5 associated with congenital heart disease and adult-onset cardiomyopathy. Circ Cardiovasc Genet 2013;6:238-47. [Crossref] [PubMed]
- Perera JL, Johnson NM, Judge DP, et al. Novel and highly lethal NKX2.5 missense mutation in a family with sudden death and ventricular arrhythmia. Pediatr Cardiol 2014;35:1206-12. [Crossref] [PubMed]
- Maury P, Gandjbakhch E, Baruteau AE, et al. Cardiac phenotype and long-term follow-up of patients with mutations in NKX2-5 Gene. J Am Coll Cardiol 2016;68:2389-90. [Crossref] [PubMed]
- Ellesoe SG, Johansen MM, Bjerre JV, et al. Familial atrial septal defect and sudden cardiac death: identification of a novel NKX2-5 mutation and a review of the literature. Congenit Heart Dis 2016;11:283-90. [Crossref] [PubMed]
- Benson DW, Sharkey A, Fatkin D, et al. Reduced penetrance, variable expressivity, and genetic heterogeneity of familial atrial septal defects. Circulation 1998;97:2043-8. [Crossref] [PubMed]
- Gutierrez-Roelens I, De Roy L, Ovaert C, et al. A novel CSX/NKX2-5 mutation causes autosomal-dominant AV block: are atrial fibrillation and syncopes part of the phenotype? Eur J Hum Genet 2006;14:1313-6. [Crossref] [PubMed]
- Shiojima I, Komuro I, Inazawa J, et al. Assignment of cardiac homeobox gene CSX to human chromosome 5q34. Genomics 1995;27:204-6. [Crossref] [PubMed]
- Pashmforoush M, Lu JT, Chen H, et al. Nkx2-5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell 2004;117:373-86. [Crossref] [PubMed]
- Lyons I, Parsons LM, Hartley L, et al. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev 1995;9:1654-66. [Crossref] [PubMed]
- Tanaka M, Chen Z, Bartunkova S, et al. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development 1999;126:1269-80. [PubMed]
- Biben C, Weber R, Kesteven S, et al. Cardiac septal and valvular dysmorphogenesis in mice heterozygous for mutations in the homeobox gene Nkx2-5. Circ Res 2000;87:888-95. [Crossref] [PubMed]
- Xu YJ, Qiu XB, Yuan F, et al. Prevalence and spectrum of NKX2.5 mutations in patients with congenital atrial septal defect and atrioventricular block. Mol Med Rep 2017;15:2247-54. [Crossref] [PubMed]
- Stallmeyer B, Fenge H, Nowak-Gottl U, et al. Mutational spectrum in the cardiac transcription factor gene NKX2.5 (CSX) associated with congenital heart disease. Clin Genet 2010;78:533-40. [Crossref] [PubMed]
- Elliott DA, Kirk EP, Yeoh T, et al. Cardiac homeobox gene NKX2-5 mutations and congenital heart disease: associations with atrial septal defect and hypoplastic left heart syndrome. J Am Coll Cardiol 2003;41:2072-6. [Crossref] [PubMed]
- Hirayama-Yamada K, Kamisago M, Akimoto K, et al. Phenotypes with GATA4 or NKX2.5 mutations in familial atrial septal defect. Am J Med Genet A 2005;135:47-52. [Crossref] [PubMed]
- Wang J, Liu XY, Yang YQ. Novel NKX2-5 mutations responsible for congenital heart disease. Genet Mol Res 2011;10:2905-15. [Crossref] [PubMed]
- Abou Hassan OK, Fahed AC, Batrawi M, et al. NKX2-5 mutations in an inbred consanguineous population: genetic and phenotypic diversity. Sci Rep 2015;5:8848. [Crossref] [PubMed]


