Early changes in coagulation profiles and lactate levels in patients with septic shock undergoing extracorporeal membrane oxygenation
Introduction
The annual incidence of sepsis, a leading cause of death worldwide, is increasing, and the mortality rate from septic shock is 25–50% (1). Extracorporeal membrane oxygenation (ECMO) has become a life-saving treatment for neonates and children with septic shock refractory to conventional treatment (2,3). However, its utility remains controversial in adult patients (4,5). In addition, ECMO is costly (6).
Disseminated intravascular coagulation (DIC) develops in 35% of patients with severe sepsis and is associated with accelerated organ failure (7,8). Data from a well-controlled clinical trial revealed abnormalities in coagulopathy, inflammation, and the levels of biomarkers of endothelial injury in patients with severe sepsis (9). Specifically, several authors have highlighted the importance of the dynamic evolution of coagulation marker levels (10,11). However, coagulopathy can also progress in patients undergoing ECMO, which is associated with blood-surface interactions and/or clotting within the ECMO device (12). Hence, we suggest that ECMO accelerates pre-existing coagulopathy in septic shock patients; the extent of coagulopathy would thus differ between survivors and non-survivors from the time at which ECMO support is commenced.
In the present study, we hypothesised that the early changes in coagulation profiles and lactate levels would be significantly associated with hospital outcomes in patients with septic shock undergoing ECMO and, therefore, that the evaluation of these parameters might be useful to guide physician decision-making in terms of ECMO treatment.
Methods
Study population
We retrospectively reviewed data from 37 patients (aged ≥18 years) with septic shock who underwent ECMO at Hallym University Sacred Heart Hospital between January 2007 and September 2015.
We followed the recommendations of the Surviving Sepsis Campaign guidelines when diagnosing and treating septic shock (13,14). ECMO was implemented for patients in whom the aetiology of septic shock was considered potentially reversible by physicians. Veno-arterial (VA) ECMO was indicated for patients with acute circulatory failure and a systolic blood pressure <80 mmHg despite adequate intravascular volume replacement and high-dose vasopressor infusion (norepinephrine >0.5 µg/kg/min), and in those with in-hospital cardiac arrest >10 min in duration despite cardiopulmonary resuscitation (CPR). However, veno-venous (VV) ECMO was first instituted for patients with septic shock associated with acute respiratory failure, with a ratio of arterial oxygen tension to inspired oxygen fraction (PaO2/FiO2) <100 on ventilation with an FiO2 of 100% and low-dose vasopressor infusion (norepinephrine <0.5 µg/kg/min). Veno-arteriovenous (VAV) ECMO was also used to treat patients with acute respiratory failure with a PaO2/FiO2 ratio <100 on ventilation with a FiO2 of 100% and high-dose vasopressor infusion (norepinephrine >0.5 µg/kg/min).
The exclusion criteria were an intracranial haemorrhage, terminal malignancy, end-stage renal disease, liver cirrhosis (Child grade > B), use of steroid or immunosuppressant agent, out-of-hospital cardiac arrest, and loss of independence in terms of the activities of daily living. However, patients were eligible when their cancers had been in complete remission for >6 months or when they were being treated with a low dose steroid (i.e., ≤10 mg/day prednisolone or equivalent). We calculated DIC scores using International Society of Thrombosis and Haemostasis (ISTH) criteria (15). This score incorporates the platelet count, D-dimer level, prothrombin time (PT), and fibrinogen level. Overt DIC was defined by a DIC score ≥5.
EMCO implantation and management
We used Capiox EBS™ (Terumo, Tokyo, Japan) or PLS (MAQUET, Hirrlingen, Germany) equipment. Both femoral veins (VV ECMO) or one femoral vein and one femoral artery (VA ECMO) were percutaneously cannulated using the Seldinger technique under fluoroscopic guidance in a cardiac catheterisation laboratory. 19-Fr (for infusion) and 21-Fr (for drainage) cannulas were placed (DLP® and Bio-Medicus®, Medtronic, Minneapolis, MN, USA; RMI®, Edward’s Life sciences LLC, Irvine, CA, USA). We placed the drainage cannula in the right femoral vein, and the infusion cannulas in the left femoral vein and right femoral artery, in VAV mode. During ECMO support, heparin or nafamostat mesilate (SK Chemicals Life Science Biz., Seoul, Korea; licensed by Torii Pharmaceutical, Tokyo, Japan) was used for anticoagulation; the target activated partial thromboplastin time (aPTT) was 60–80 s. The target haematocrit and platelet counts were >35% and >50,000–80,000/mm3, respectively. Patients received antithrombin (AT) III when the initial AT III level was <70%, with a loading dose of 2,000 IU followed by a maintenance dose of 500 IU every 6 h for 3 days. Continuous renal replacement therapy (CRRT) was commenced if a patient exhibited progressive oliguria (i.e., urine output <0.5 cc/kg/h for >6 h).
Data collection and outcomes
The following data were obtained by a retrospective review of the medical records: demographic characteristics (age, sex, and body mass index), comorbidities, the cause of sepsis, and the need for CPR before or during ECMO. Pre-ECMO laboratory data, vasoactive inotropic scores (16), and severity-of-illness scores [Simplified Acute Physiology Scores (SAPS) II and sequential organ failure assessment (SOFA) scores] were calculated. Coagulation data included the platelet count; PT; aPTT; and D-dimer, fibrinogen, and AT III levels at each of the three time points [day 0 (within 24 h of ECMO initiation), day 1 (24 h after initiation), and day 2 (48 h after initiation)]; we also calculated DIC scores.
The primary outcomes were the impact of the pre-ECMO DIC score on hospital death, and differences in the evolution of the lactate levels and coagulation profiles between survivors and non-survivors during the early period of ECMO support. The secondary outcomes were the associations between DIC scores and lactate levels, and complications in the pre-ECMO overt-DIC vs. the non-overt-DIC groups.
Statistical analysis
All results are presented as numbers with percentages for categorical variables, and as medians with interquartile ranges for continuous variables. The Mann-Whitney U test or a repeated measure analysis of variance (ANOVA) was used to compare continuous variables, and the chi-squared or Fisher’s exact test was employed to compare categorical variables. Spearman’s method was used to seek correlations between DIC scores and lactate levels.
A logistic regression analysis was performed using covariates significant (P<0.05) on the univariate analysis to identify independent risk factors for hospital mortality. In terms of ECMO type, we combined the VA and VAV ECMO groups into one group (i.e., we compared the VV type with the other types) in the multivariate analysis. Receiver operating characteristic (ROC) curves were drawn to determine the ability of pre-ECMO variables, including the DIC score, to predict hospital death. IBM SPSS version 22.0 software for Windows (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Results
Baseline characteristics
During the study period, 207 patients underwent ECMO support; of these, 39 were treated because of septic shock. Two patients were finally excluded because of necrotising pancreatitis and gastrointestinal bleeding, respectively. The median patient age was 51.0 years (35.5–64.5 years) and 25 patients were male (Table 1). The most common causes of septic shock were pneumonia (n=17) and intra-abdominal infection (n=5). Among the organisms (n=23) identified, Klebsiella pneumoniae and methicillin-sensitive Staphylococcus aureus were the most prevalent (Table 2); 51.4% of patients had bacteremia. Cardiac arrest occurred in 18 patients (ECPR, n=8); 16 underwent echocardiography before ECMO commencement. VA ECMO was performed on 26 patients and VV ECMO on 8 (VAV ECMO on 3). During the first 48 h of ECMO, the median ECMO flow was 4.3 L/min (3.8–4.6 L/min) in the VA and VAV groups and 4.8 L/min (4.0–5.1 L/min) in the VV ECMO group. CRRT was given to 36 patients during ECMO support.
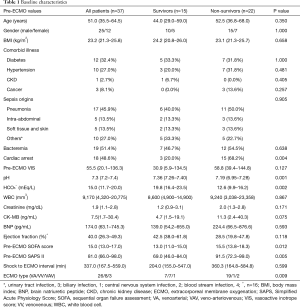
Full table
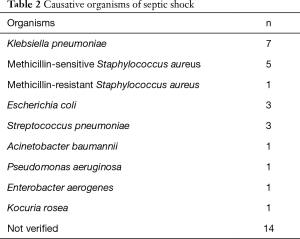
Full table
Evolution of the coagulation profiles in the pre-ECMO overt-DIC and non-overt-DIC groups
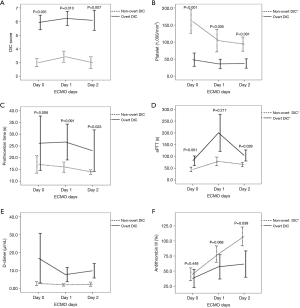
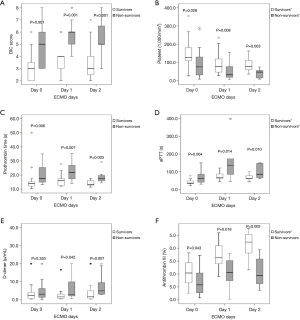
The overall incidences of overt DIC on days 0, 1, and 2 were 40.5% (15/37), 48.1% (13/27), and 47.8% (11/23), respectively. Patients with pre-ECMO (day 0) overt DIC had higher DIC scores at all three time points compared to those with non-overt DIC, but the DIC scores did not change significantly during the period of ECMO support in either group (Figure 1). Among the individual parameters, platelet count, aPTT, and AT III level changed significantly only in patients with non-overt DIC.
The coagulation profiles of survivors and non-survivors
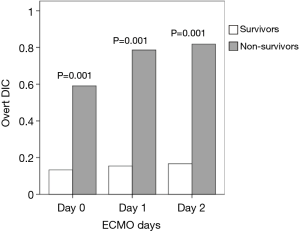
Of the 37 patients, 15 (40.5%) survived to discharge. Before and during ECMO treatment, the incidence of overt DIC and the DIC score were significantly higher among non-survivors than survivors (Figures 2,3). However, no significant temporal change in DIC score was found during the early ECMO period in either group. In terms of individual parameters (i.e., platelet count, aPTT, AT III level, PT, and D-dimer level), survivors exhibited better coagulation profiles than non-survivors (Figure 3), but the fibrinogen levels did not differ between the groups (data not shown).
Evolution of lactate levels and their correlations with DIC scores
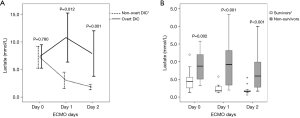
The lactate level trajectories differed considerably between patients with pre-ECMO overt DIC and those with non-overt DIC; lactate levels decreased significantly only in the latter group (Figure 4A). Lactate levels also decreased significantly in survivors, but not non-survivors (Figure 4B). The DIC scores correlated with lactate levels on both day 1 and day 2 (r=0.825, P<0.001 and r=0.667, P=0.001, respectively; vs. r=0.167, P=0.322 on day 0).
Complication rates in the pre-ECMO overt-DIC and non-overt-DIC groups
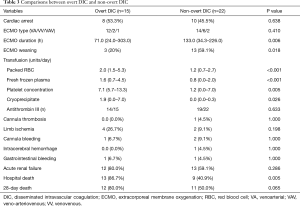
Patients with pre-ECMO overt DIC exhibited a lower rate of ECMO weaning (20.0% vs. 59.1%, P=0.018) and higher hospital mortality (86.7% vs. 40.9%, P=0.005) compared to those with non-overt DIC. In particular, the number of transfusions required (red blood cells, platelet concentrate, fresh frozen plasma, and cryoprecipitate) were significantly higher in patients with overt DIC (Table 3). The rates of complications, including cannula bleeding, limb ischaemia, and gastrointestinal bleeding, did not differ between the two groups.
Full table
DIC scores in the VA-ECMO and VV-ECMO groups
After excluding patients suffering cardiac arrest, the DIC scores were significantly higher in the VA ECMO group (n=13) than in the VV ECMO group (n=6) [5.0 (3.5–6.0) vs. 2.5 (2.0–3.0), P=0.001 on day 0; 4.5 (4.0–6.0) vs. 3.0 (2.0–4.5), P=0.042 on day 1; 4.5 (3.9–7.0) vs. 3.0 (2.0–3.0), P=0.005 on day 2). However, after ECMO was commenced, no temporal changes in the scores were observed in either group. Patients receiving VV ECMO tended to have better coagulation profiles than those receiving VA ECMO (data not shown).
Multivariate analysis of factors affecting hospital mortality
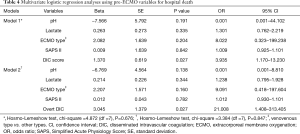
In a univariate analysis using pre-ECMO variables, the following parameters were significant (P<0.05): cardiac arrest, ECMO type (VV vs. other types), DIC score, overt DIC, platelet count, PT, aPTT, AT III level, pH, bicarbonate and lactate levels, SAPS II, and SOFA score. Of these, considering the overlapping meaning between the variables, we finally included five variables (pH, ECMO type, pre-ECMO DIC score, lactate level, and SAPS II) in a multivariate analysis (Table 4). In the final model (Model 1), pre-ECMO DIC score was an independent risk factor for hospital death [odds ratio (OR), 3.935; 95% confidence interval (CI), 1.170–13.230]. When we replaced pre-ECMO DIC score with the categorical variable (i.e., pre-ECMO overt DIC) (Model 2), we also found a significant association with the risk of hospital death.
Full table
ROC curves predicting hospital mortality
We measured the areas under the curves (AUCs) of various pre-ECMO parameters (Table 5 and Figure 5); the pre-ECMO DIC score was a good predictor of hospital death (AUC, 0.815; 95% CI, 0.676–0.954). However, of the variables investigated, pre-ECMO DIC score plus lactate level was the best predictor (0.897; 0.771–0.987); the optimal cut-off was 9.35 (sensitivity, 81.8%; specificity, 73.3%; positive predictive value, 81.8%; and negative predictive value, 73.3%). The hospital mortality of patients with scores >9.35 was three-fold higher than that of others (81.8% vs. 26.7%, P=0.001).
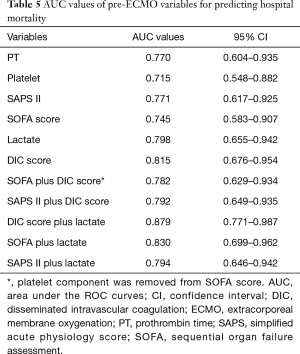
Full table

Discussion
We report several interesting findings. First, the pre-ECMO DIC score was an independent risk factor for hospital death in septic shock patients undergoing ECMO. Second, the coagulation profiles (including DIC scores) and lactate levels, differed significantly between survivors and non-survivors both before and during ECMO support. Finally, the pre-ECMO DIC score plus lactate level variable was the best predictor of hospital death, and patients with combined scores >9.35 had a three-fold higher hospital mortality rate than did others.
Recently, some experts have claimed that coagulation is an important component of the innate immune system and can contribute to pathogen clearance in sepsis by creating a physical barrier (17,18). Although physicians should be aware of this paradigm shift to understand more clearly the role played by DIC in sepsis, it has long been known that both inflammation and coagulation play important roles in the development of DIC in septic shock patients, and that DIC development is associated with poorer outcomes.
Various DIC diagnostic criteria have been established by different groups (15,19,20). In the present study, we used ISTH criteria and found that >40% of patients developed overt DIC during the early period of septic shock. Previously, Park et al. reported that the incidence of overt DIC was 27.9–30.1% in the early period of sepsis, and that changes in DIC scores were associated with increased hospital mortality (21). Other studies also found that worsening scores (‘composite coagulopathy scores’ and ‘simple evolving DIC scores’) reflected poor patient outcomes (10,11). However, ECMO per se is known to promote coagulopathy (22). In the present study, we hypothesised that pre-existing coagulopathy would be accelerated in sepsis patients upon the commencement of ECMO treatment. However, based on our results, ECMO did not have a strong effect on pre-existing coagulopathy in patients with septic shock. We found that DIC scores, PT, and D-dimer did not change even after ECMO was commenced; only the platelet count decreased significantly in the non-overt DIC group (or both in survivors and non-survivors). These findings may be associated with the beneficial effect of ECMO treatment on organ perfusion (i.e., organ function) (23), considering the association between organ dysfunction and the development of DIC. However, this needs further research.
The development of overt DIC or a high DIC score prior to the commencement of ECMO was associated with a higher rate of hospital mortality. Particularly, such patients required more transfusions during ECMO support than did those with non-overt DIC (or lower DIC scores); this may partly explain their poor outcomes. However, again, the DIC scores did not subsequently change, and the difference between patients with overt DIC and those with non-overt DIC did not vary, during the 48 h of ECMO support. This supports the significant association evident in the present study between pre-ECMO DIC score (or overt DIC) and hospital death.
The overall survival-to-discharge rate was slightly higher (40.5%) in our study than in previous studies of patients with septic shock undergoing ECMO (27.8% in the work of Cheng et al. (24), 21.9% in that by Park et al. (4), and 15.4% in the study of Huang et al. (25). This may be partly attributable to the short interval between the development of septic shock and ECMO implementation (337.0 min), which was less than in previous studies (4,26), indicating that a prompt decision to implement ECMO may be associated with more favourable outcomes. Another explanation may be our inclusion of VV ECMO patients. Although VA ECMO is primarily used to support patients with septic shock haemodynamically, experts recommend that VV ECMO should be considered first in patients with pneumonia-induced septic shock and those with sepsis-induced acute respiratory distress syndrome (27). Although heterogeneity of the study population may be an issue in our study, sepsis per se is a complex disease (i.e., disease heterogeneity) (28). Our primary aim of ECMO was to improve organ perfusion (i.e., tissue oxygenation) for patients in septic shock. Our decision on whether to perform VV ECMO or VA ECMO is based on the patient’s oxygenation and haemodynamics. Hence, we selected different treatment modalities depending on the patient’s condition while patients were in septic shock. Although we acknowledge the problem of heterogeneity, inherent biological and clinical heterogeneity of sepsis should be considered (28).
Several previous studies found that the pre-ECMO lactate level was associated with hospital mortality (4,29,30). In our study, we also found that changes in lactate levels differed markedly between the pre-ECMO overt-DIC and non-overt-DIC groups (and between survivors and non-survivors). Lactate levels fell notably in the pre-ECMO non-overt-DIC group and survivors. This is consistent with the recent report by Bonizzoli et al., where lactate levels and their clearance during the early period of ECMO treatment were a prognostic factor in patients with refractory acute respiratory distress syndrome receiving VV ECMO (29). However, in our study, the majority of patients were receiving VA ECMO due to circulatory shock. More interestingly, we found that the combined score of pre-ECMO DIC score plus lactate level had the best AUC value of all parameters tested; the optimal cut-off (i.e., 9.35) afforded good sensitivity and specificity. Our data suggest that pre-ECMO combined scores >9.35 might indicate a poor hospital outcome in patients with sepsis who are considered for ECMO. Although a complex score could be difficult to apply in clinical practice, this is the first report to show that the pre-ECMO DIC score plus lactate level is predictive in septic shock patients on ECMO, and this finding deserves particular attention.
Several limitations must be considered when interpreting our results. First, our patient numbers were small, and the retrospective nature of the work may have introduced unidentified bias. Second, a small portion of patients were treated via VV or VAV ECMO despite septic shock, which could be associated with the heterogeneity of the study population. Third, the study period when data were collected was long. Some changes in the treatment strategies for patients may have occurred. Finally, we could not collect detailed echocardiographic data, which would have strengthened our findings. In particular, we used our institutional strategy for commencing ECMO in septic shock patients, which limited generalisability. Therefore, this should be considered when interpreting the data. However, to the best of our knowledge, few data are available for septic shock patients receiving ECMO; this is the first study to describe the evolution of the coagulation profiles and lactate levels in such patients.
Conclusions
The pre-ECMO DIC score was a significant risk factor for hospital death in patients with septic shock. During the 48 h of ECMO support, the coagulation profiles, DIC scores, and lactate levels differed significantly between survivors and non-survivors. Furthermore, the combined variable pre-ECMO DIC score plus lactate level had the best AUC value, and combined scores >9.35 might indicate a poor hospital outcome.
Acknowledgements
The authors want to thank Young Soo Ju (Department of Occupational and Environmental Medicine, Hallym University Sacred Heart Hospital) for his contribution to statistical analysis.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The present study was approved by the Institutional Review Board of Hallym Medical Center (IRB No. 2016-I155). Informed consent was waived because this was a retrospective study.
References
- Beck V, Chateau D, Bryson GL, et al. Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Timing of vasopressor initiation and mortality in septic shock: a cohort study. Crit Care 2014;18:R97. [Crossref] [PubMed]
- Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med 2009;37:666-88. [Crossref] [PubMed]
- Sivarajan VB, Almodovar MC, Rodefeld MD, et al. Pediatric extracorporeal life support in specialized situations. Pediatr Crit Care Med 2013;14:S51-61. [Crossref] [PubMed]
- Park TK, Yang JH, Jeon K, et al. Extracorporeal membrane oxygenation for refractory septic shock in adults. Eur J Cardiothorac Surg 2015;47:e68-74. [Crossref] [PubMed]
- Gabel E, Gudzenko V, Cruz D, et al. Successful use of extracorporeal membrane oxygenation in an adult patient with toxic shock-induced heart failure. J Intensive Care Med 2015;30:115-8. [Crossref] [PubMed]
- Hsu CP, Lee WC, Wei HM, et al. Extracorporeal membrane oxygenation use, expenditure, and outcomes in taiwan from 2000 to 2010. J Epidemiol 2015;25:321-31. [Crossref] [PubMed]
- Bakhtiari K, Meijers JC, de Jonge E, et al. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med 2004;32:2416-21. [Crossref] [PubMed]
- Dhainaut JF, Yan SB, Joyce DE, et al. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost 2004;2:1924-33. [Crossref] [PubMed]
- Kinasewitz GT, Yan SB, Basson B, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism Crit Care 2004;8:R82-90. [ISRCTN74215569]. [Crossref] [PubMed]
- Kinasewitz GT, Zein JG, Lee GL, et al. Prognostic value of a simple evolving disseminated intravascular coagulation score in patients with severe sepsis. Crit Care Med 2005;33:2214-21. [Crossref] [PubMed]
- Dhainaut JF, Shorr AF, Macias WL, et al. Dynamic evolution of coagulopathy in the first day of severe sepsis: relationship with mortality and organ failure. Crit Care Med 2005;33:341-8. [Crossref] [PubMed]
- Esper SA, Levy JH, Waters JH, et al. Extracorporeal membrane oxygenation in the adult: a review of anticoagulation monitoring and transfusion. Anesth Analg 2014;118:731-43. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med 2008;34:17-60. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637. [Crossref] [PubMed]
- Taylor FB Jr, Toh CH, Hoots WK, et al. Scientific Subcommittee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis; Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost 2001;86:1327-30. [Crossref] [PubMed]
- Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010;11:234-8. [Crossref] [PubMed]
- Fiusa MM, Carvalho-Filho MA, Annichino-Bizzacchi JM, et al. Causes and consequences of coagulation activation in sepsis: an evolutionary medicine perspective. BMC Med 2015;13:105-13. [Crossref] [PubMed]
- van der Poll T, Herwald H. The coagulation system and its function in early immune defense. Thromb Haemost 2014;112:640-8. [Crossref] [PubMed]
- Kobayashi N, Maekawa T, Takada M, et al. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the Research Committee on DIC in Japan. Bibl Haematol 1983;49:265-75. [PubMed]
- Gando S, Iba T, Eguchi Y, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med 2006;34:625-31. [Crossref] [PubMed]
- Park JY, Park S, Park SY, et al. Day 3 versus Day 1 disseminated intravascular coagulation score among sepsis patients: a prospective observational study. Anaesth Intensive Care 2016;44:57-64. [PubMed]
- Weingart C, Lubnow M, Philipp A, et al. Comparison of Coagulation Parameters, Anticoagulation, and Need for Transfusion in Patients on Interventional Lung Assist or Veno-Venous Extracorporeal Membrane Oxygenation. Artif Organs 2015;39:765-73. [Crossref] [PubMed]
- Kang JH, Choi BH, Moon KM, et al. Beneficial effect of extracorporeal membrane oxygenation on organ perfusion during management of the unstable brain-dead donor: a case series. Transplant Proc 2016;48:2458-60. [Crossref] [PubMed]
- Cheng A, Sun HY, Lee CW, et al. Survival of septic adults compared with nonseptic adults receiving extracorporeal membrane oxygenation for cardiopulmonary failure: a propensity-matched analysis. J Crit Care 2013;28:532.e1-10. [Crossref] [PubMed]
- Huang CT, Tsai YJ, Tsai PR, et al. Extracorporeal membrane oxygenation resuscitation in adult patients with refractory septic shock. J Thorac Cardiovasc Surg 2013;146:1041-6. [Crossref] [PubMed]
- Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011;365:1905-14. [Crossref] [PubMed]
- Bréchot N, Luyt CE, Schmidt M, et al. Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Crit Care Med 2013;41:1616-26. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Bonizzoli M, Lazzeri C, Cianchi G, et al. Serial lactate measurements as a prognostic tool in venovenous extracorporeal membrane oxygenation support. Ann Thorac Surg 2017;103:812-8. [Crossref] [PubMed]
- Dennis M, McCanny P, D'Souza M, et al. Extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest: A multicentre experience. Int J Cardiol 2017;231:131-6. [Crossref] [PubMed]


