Short-term one-lung ventilation does not influence local inflammatory cytokine response after lung resection
Introduction
One-lung ventilation (OLV), routinely used in thoracic surgery to achieve optimal surgical exposure, has its own particular features and problems as compared to two-lung ventilation (TLV). OLV may cause vascular congestion and an inflammatory response resulting in potentially harmful effects on the pulmonary parenchyma (1). For years, hypoxia has been the most important issue during OLV, and its incidence is about 5–10% (2). During OLV, the perfusion/ventilation ratio in the non-dependent lung changes. An auto-compensatory response and hypoxic pulmonary vasoconstriction (HPV) occurs, distributing pulmonary capillary blood flow to areas of high-oxygen availability to optimize gas exchange (3). This mechanism causes extensive pulmonary vasoconstriction and associated vascular congestion (4). Furthermore, OLV determines the activation of the inflammatory response with a release of inflammatory mediators by the bronchial epithelium; this occurs both in the dependent and non-dependent lung (5), but mostly in the ventilated one (6). The most important issue concerning OLV is actually the development of postoperative respiratory complications, such as acute lung injury and acute respiratory distress syndrome (7); in fact, ALI and ARDS are the leading causes of death after lung resection. Elevated VT and airway pressure are associated with an increased incidence in these complications (8). For years, the use of large tidal volume (VT 8–10 mL/kg), zero end-expiratory pressure (ZEEP), and a high fraction of inspired oxygen (FiO2) during OLV had been recommended in order to not increase vascular resistance, to limit transpulmonary shunt and prevent the onset of atelectasis in the ventilated lung, ensuring arterial oxygenation and the elimination of CO2 (9,10). Recent studies have questioned the safety of the conventional ventilation (CV) strategy during OLV, due to a decrease in oxygenation, an increase in inflammatory products and reactive oxygen species resulting in damage to lung tissue (11,12). Several authors have demonstrated that the using a protective ventilation (PV) strategy with a small VT (4–6 mL/kg) reduces the incidence of postoperative ALI and ARDS in patients undergoing lung resection (13) and decreases the systemic inflammatory cytokine response as compared to CV (14,15). Since several studies evaluated the level of a systemic inflammatory response, that also depends on the extent of surgical trauma, lung cancer and comorbidities, the attribution of OLV to inflammatory response is not yet clear (16). It is assumed that OLV may initiate a pro-inflammatory response in the alveolar compartment of the dependent lung, nevertheless, pleural fluid measurement and bronchoalveolar lavage (BAL) yield disputable data (17). Thus far, most studies compared high VT without PEEP to low VT and PEEP; therefore, it is not known whether mechanical ventilation with PEEP and high VT could induce an inflammatory response. The aim of this study was to assess the local inflammatory cytokine response of the dependent lung and determine the causative role of VT in lung injury during OLV in patients undergoing lung resection. The secondary aim was to evaluate the possible correlation between inflammatory cytokine levels and postoperative respiratory complication occurrence and length of stay.
Methods
Patients and methods
The study was approved by the Bioethics Committee of Sapienza University of Rome (No. 3722_2015), and informed consent was obtained from each patient in writing prior to enrolment. From November to December 2016, thirty patients scheduled for elective lobectomy or wedge resection via thoracotomy were included in the study. Exclusion criteria were emergency surgery, pregnancy, patient refusal to give consent, inability to give consent, age ≤18 years and ASA ≥ IV. Two patients were excluded from the study due to changes in planned surgery and refusal to give consent. Preoperative data such as age, sex, weight, height, body mass index (BMI) and predicted body weight (PBW) were analyzed. The type of surgery, ASA score, forced expiratory volume in the first second (FEV1, % predicted), comorbidities and preoperative ABG (arterial blood gas) were considered. The intraoperative data such as the duration of OLV, total fluid infusions, VT, peak inspiratory pressure (P peak), plateau pressure (P plateau), compliance, end-tidal partial pressure of carbon dioxide (EtCO2), respiratory rate (RR), peripheral oxygen saturation (SpO2), systolic, diastolic, mean blood pressure and ABG were analyzed.
Anesthesia and surgery
Before surgery, patients received premedication with midazolam 0.03 mg/kg, ondansetron 8 mg, ketorolac tromethamine 30 mg, dexamethasone 4 mg and pantoprazole 40 mg intravenously. Anesthesia was induced intravenously using propofol 1.5–2 mg/kg, fentanyl 2 µg/kg and ketamine 0.3 mg/kg. Orotracheal intubation was performed with a double lumen tube (DLT) after administration of rocuronium bromide 0.6 mg/kg. The correct positioning of the DLT was evaluated by fiberoptic bronchoscopy (FOB) following endotracheal intubation, and then, once the patient was positioned on lateral decubitus. Maintenance of general anesthesia was achieved with desflurane (minimum alveolar concentration MAC-1) and continuous infusion of remifentanil, using a target-controlled infusion (TCI) system in effect-site target mode with an Alaris® PK Syringe Pump. The target effect-site concentration of remifentanil was calculated by a Minto pharmacokinetic set. Ventilation was performed in volume-controlled mode, with 5 cmH2O PEEP, FiO2 of 0.5, a 1:2 inspiratory-expiratory ratio and a RR of 12–18 breaths/min in order to maintain an end-tidal concentration of CO2 <40 mmHg. Prior to the opening of the chest wall and just after the positioning of the patient in lateral decubitus, OLV was immediately started with a VT calculated according to PBW and selected at the discretion of the operating room anesthesiologist. In the case of desaturation (SpO2 <90%), the DLT position would be verified with an FOB before progressively increasing the FiO2 by 10%; alveolar recruitment maneuvers would be made through manual inflations for 10–40 seconds to reach a peak pressure of 30–40 cm H2O. The maximum P peak on volume-controlled ventilation would be set at 35 cmH2O. When this value was exceeded, volume-controlled ventilation would be changed to pressure-controlled ventilation, with a setting providing the same VT. Intraoperative monitoring would include a three-lead ECG, invasive blood pressure (IBP) continuously recorded via a-line, oxygen saturation by pulse oximetry (SpO2), and expired CO2. Arterial blood gas (ABG) analysis was performed every 30 minutes from the start of OLV. A moderate intravenous fluid management of 5–6 mL/kg/h was used. The surgical approach was based on a muscle-sparing mini-thoracotomy. At the end of surgery, an intrapleural, intercostal nerve block was performed using 20 mL of ropivacaine 0.5%. The chest tube insertion point and the thoracic wound were also infiltrated with 10 mL of ropivacaine 0.5%. Postoperative analgesia was delivered using a 24-hour continuous infusion of tramadol and ketorolac with an elastomeric pump.
BAL analysis
BAL was performed selectively from the dependent lung immediately before and at the end of OLV (before start two lungs ventilation) by instilling 20 mL of sterile isotonic saline in the segments of the lower lobe of the dependent lung. During bronchoscopy, the FiO2 was kept at 1.0.
BAL was centrifuged (200 g, 4 °C, 10 min) and aliquots from the clear supernatant were stored at 80 °C for analysis. The levels of pro-inflammatory interleukins (IL-1α, IL-1β, IL-6, IL-8), tumor necrosis factor-alpha (TNFα), vascular endothelial growth factor (VEGF), endothelial growth factor (EGF), monocyte chemoattractant protein-1 (MCP), and anti-inflammatory cytokines, such as interleukins (IL-2, IL-4, IL-10) and interferon (IFN-γ), were evaluated simultaneously using cytokine & growth factor arrays from Evidence Investigator Biochip Array technology® (Randox Laboratories, Crumlin, UK) according to the manufacturer’s instructions. The sensitivities of the test kits were as follows: IL-1α: 0.8 pg/mL, IL-1β: 1.6 pg/mL, IL-6: 1.2 pg/mL, IL-8: 4.9 pg/mL, TNFα: 4.4 pg/mL, VEGF: 14.6 pg/mL, EGF: 2.9 pg/mL, MCP-1: 13.2 pg/mL, IL-10: 1.8 pg/mL, IL-2: 4.8 pg/mL, IL-4: 6.6 pg/mL, IL-10: 1.8 pg/mL, IFN-γ: 3.5 pg/mL.
Subgroup analysis
To analyze the VT set during OLV, each patient received 5–10 mL/kg and two subgroups can be distinguished: a CV subgroup, comprised of 13 patients, received 8–10 mL/kg and a PV subgroup, comprised of 15 patients, received 5–7 mL/kg.
Statistics
The calculation of the sample size was based on previous studies (11,12,18,19) which took into account the tested hypothesis that cytokines would vary more than 25% after OLV with a power of 0.9; thus, a sample size of 30 patients was required. The Shapiro-Wilk test was used to assess the normality of distributions. Mean values and standard deviation (SD) were determined by Student’s t-test for each quantitative variable. Fisher exact test was applied for qualitative variables. The Mann-Whitney U-test was used when the distribution was non normal. Parameters with values deviating from a normal distribution were analyzed by the median lower quartile (Q1) and upper quartile (Q3). P values <0.05 were considered significant. The data was analyzed using the SPSS v20.0 software (SPSS Inc., Chicago, IL, USA).
Results
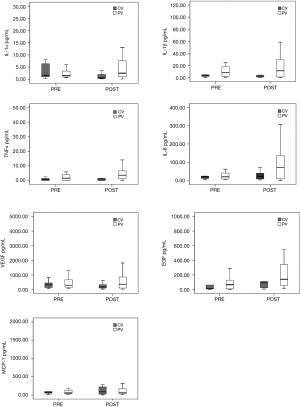
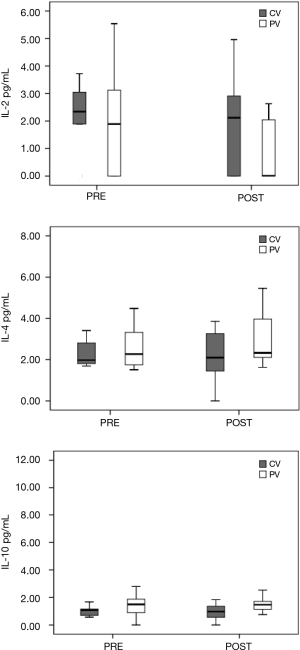
The study included 28 consecutive patients (14 females and 14 males), from 52–83 years of age, undergoing elective surgery for lung resection. There were no statistically significant differences in the demographic or clinical data among the patients and between the two ventilatory subgroups as illustrated in Table 1. Shown in Table 2 is the comparative intraoperative data for (I) the overall population analyzed thus far, (II) the CV subgroup, and (III) the PV subgroup. During mechanical ventilation, a higher ventilator rate was required to achieve an optimal EtCO2 range at 30 minutes of OLV in the PV subgroup as compared to the CV subgroup (RR 16.27±2.34 in the PV subgroup, 11.64±1.91 in the CV subgroup; P=0.001). At the same evaluation point, EtCO2 and PaCO2 were higher in the PV subgroup (EtCO2 30.00±4.92 mmHg in the CV subgroup, EtCO2 35.00±5.38 mmHg in the PV subgroup; P=0.036; PaCO2 39.36±4.52 mmHg in the CV subgroup, PaCO2 48.80±4.78 mmHg in the PV subgroup; P=0.001). Peak airway pressure was lower during mechanical ventilation with a small VT at 15 and 30 minutes in the PV subgroup (P peak 15 min 22.00±8.21 cmH2O, and P peak 30 min 26.00±7.04 cmH2O in the PV subgroup; P peak 15 min 29.00±5.12 cmH2O and P peak 30 min 30.00±4.52 cmH2O in the CV subgroup; respectively P=0.016 and P=0.027). Plateau pressure was lower in the PV subgroup at 15 minutes (P plateau 15 min 16.00±6.31 cmH2O in the PV subgroup; P plateau 15 min 23.50±4.7 cmH2O in the CV subgroup; P=0.014). Peripheral oxygen saturation at 30 minutes was higher in the CV subgroup (SpO2% 30 min 95.00±2.98 in the PV subgroup; SpO2% 30 min 98.00±1.86 in the CV subgroup; P=0.027). No postoperative respiratory complications were recorded, and no patient required postoperative admission to the ICU. The average length of a hospital stay was 4.19±1.69 days with no significant differences detected in PV patients as compared to CV patients (mean 4.00±1.41 days in PV subgroup vs. mean 4.45±2.07 days in the CV subgroup). Cytokine BAL levels are shown in Figures 1 and 2. The IFN-γ levels were below the detection threshold in 36/56 samples, whereas the IL-8 levels were above the upper limit of detection in 30/56 samples; for this reason, no further statistical analysis was performed for these cytokines. The cytokine levels measured in BAL after OLV did not significantly increase or decrease and the levels were not appreciably different between the two subgroups.
Full table
Full table
Discussion
In our study, designed to evaluate the effects of OLV on local cytokine inflammatory response, we did not observe consistent differences in pro-inflammatory and anti-inflammatory levels of cytokine in BAL. Our findings, therefore, appear to be in contrast with previous data indicating that OLV initiates a pro-inflammatory response in the alveolar compartment of the dependent lung and that the use of higher airway pressures and VT during OLV is associated with the development of ALI (11,20,21).
Mechanical ventilation may exacerbate or initiate lung injury, processes defined as ventilator-associated or ventilator-induced lung injury (VALI and VILI) (20). OLV may be an exemplary model of VILI, because OLV itself is characterized by a diminished available lung volume for ventilation, atelectasis and impaired oxygenation which result in the exposure of the ventilated lung to volutrauma and atelectrauma (22). OLV is a non-physiologic condition that can result in histologic lung injury, involving the entire alveolar-capillary unit (7) and inducing an inflammatory response in both the non-dependent and dependent lungs, but mostly in the ventilated one (5). The use of a larger VT and higher airway pressures during OLV is associated with the development of ALI and ARDS (8). These are well-known leading causes of mortality after lung resection and significantly reduce 1-year survival rates (56% vs. 92%) (11). The study of Yang et al. showed that the application of a small VT and PEEP was associated with a lower incidence of postoperative lung dysfunction and satisfactory gas exchange compared with the traditional large VT ventilation (13). Schilling et al. (17) demonstrated that OLV with a large VT may promote the production and release of pro-inflammatory substances, such as TNF-alpha and Intercellular Adhesion Molecule 1 (ICAM-1) in the dependent lung, but they did not correlate the level of these molecules with patient outcomes and clinical data. Michelet et al. (14) proved that PV strategy diminishes the pro-inflammatory systemic response and the release of IL-1, IL-6, and IL-8 after a prolonged period of OLV and ensures better oxygenation and a shorter duration of postoperative ventilation.
Certain data suggests that mechanical ventilation appears to induce no inflammation in normal lungs, but may increase lung inflammation in pre-injured or infected lungs. Tremblay et al. (23) found that injurious mechanical ventilation causes elevated production of cytokines in animals pretreated with intravenous lipopolysaccharides, whereas in the absence of this inflammatory co-stimulus, there was no induction of TNFα and IL-6. In rats without lung injury, mechanical ventilation with a VT set at 10 mL/kg did not affect cytokine release [IL-1α, IL-1β, IL-6, macrophage inflammatory protein (MIP) 2, and TNFα] in BAL fluid as compared to that of spontaneous breathing (24). On the other hand, in a rat model with hydrochloric acid instillation–induced lung injury, mechanical ventilation with a VT of 16 mL and ZEEP resulted in a marked increase of TNFα and MIP-2 as compared to a VT of 9 mL and 5 cmH2O PEEP (25). Wrigge et al. (26) demonstrated that mechanical ventilation with a large VT and ZEEP did not result in higher cytokine levels as compared to PV strategies and that previous lung damage seems to be a prerequisite for the release of cytokines.
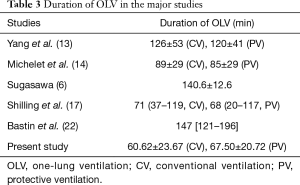
In our study, the application of PEEP may have played a protective role against inflammation. Thus far, no studies have demonstrated a definitive, specific advantage of a small VT ventilation per se in the absence of other protective ventilatory strategies as in the application of PEEP, airway pressure limitation and recruitment maneuvers. It is not yet clear, therefore, which ventilator parameter is most likely to prevent respiratory complications. Treschan et al. (27) demonstrated that there are no differences in postoperative pulmonary function between large and small VT regimens, both employing equally moderate levels of PEEP. Other studies demonstrated an association between small intraoperative VT with minimal PEEP and an increased risk of mortality and postoperative complications: low PEEP appears to be insufficient to stabilize alveoli, reduce alveolar strain, and prevent atelectasis (28,29). Application of PEEP is essential to safeguard the lung in surgical patients ventilated with both small and large VT, by improving lung function and reducing the risk of postoperative complications after OLV (13,14,30). In light of the current evidence, it could be suggested that a small VT does not in itself prevent postoperative respiratory complications without adequate PEEP, and it is interesting to note that in the most important studies, the duration of OLV was longer than that used in the present study (Table 3). The duration of OLV, in fact, is one of the primary factors in determining a pulmonary inflammatory response, and a significant correlation between a pulmonary inflammatory response and the duration of OLV has been demonstrated by several studies: De Conno et al. showed a positive correlation between cytokines release and OLV duration (31); Tekinbas et al. (32) demonstrated that, in a rat model, lung tissue myeloperoxidase activity, alveolar edema and infiltration with inflammatory cells increased in function to OLV time. These findings were also supported by Misthos et al. (33) which showed that the degree of oxidative stress was also related to the duration of OLV. The use of short-term OLV is the probable explanation for the absence of the production of inflammatory mediators in our experiment, which ultimately begins after an hour of OLV. The duration of OLV seems to be one of the major factors influencing a patients’ outcome, aside from the quality of postoperative analgesia and intraoperative fluid therapy, in positive correlation with the length of an ICU stay (34). If put into practice, approaches and techniques that would achieve the optimization of these factors could improve patient outcome and reduce critical care resources required for patients undergoing thoracic surgery (30).
Full table
Another factor influencing the pro-inflammatory response during OLV is the type of general anesthesia used. Volatile anesthetics have been shown to induce dose and time-dependent immune-modulatory effects (35). Desflurane and sevoflurane suppress local alveolar cytokine pro-inflammatory release in the ventilated lung after OLV, while propofol seems not to produce this advantageous effect (36). In particular, volatile anesthesia with desflurane attenuates the immune response and causes, as compared to propofol, a minor alveolar release of inflammatory mediators such as ICAM-1, polymorphonuclear (PMN) A elastase, IL-8 and IL-10 (37). Desflurane at 1 MAC concentration produces bronchodilator effects, similar to that of sevoflurane and isoflurane, inducing a reduction of peak inspiratory pressure and an increase in dynamic compliance, which implies the protective effects on mechanical ventilation (38). In the present study, the choice of a volatile anesthetic as opposed to an intravenous one (in addition to the use of short term OLV) may have succeeded in preventing the activation of a local inflammatory cytokine response.
The use of less invasive surgical techniques and advanced anesthesia management in order to improve surgical outcomes is also the basis of fast track surgery programs (39,40). The focus of future studies will be to advance towards the standardization of fast track protocols in order to guarantee less invasive surgical techniques, (including nerve-sparing thoracotomy, muscle-sparing incisions and mini-thoracotomy), as well as effective anesthetic management, in the hope of achieving a perioperative negative fluid input balance through the use of restrictive fluid therapy and regional analgesic techniques, such as intrapleural intercostal nerve block. According to published findings, the use of an intrapleural intercostal nerve block in a mini-thoracotomy reduces postoperative pain and contributes to improvement in postoperative outcomes after major pulmonary resection (41).
The IFN-γ levels were below the detection threshold in 36/56 samples, whereas the IL-8 levels were above the upper limit of detection in 30/56 samples. To our knowledge, no studies evaluated the IFN-γ concentration in BAL during OLV, so we cannot speculate about the significance of IFN-γ values below the limit of detection. Concerning the recorded IL-8 concentration above range of detection, this value agrees with BAL concentration of IL-8 reported in literature (17,31). Unfortunately, due to the multiplexing form of the used analysis kit, we cannot measure IL-8 on diluted samples. Thus, IFN-γ and IL-8 were excluded from further analysis.
During mechanical ventilation, a higher ventilator rate at 30 minutes of OLV (P=0.001) was required in the PV subgroup as compared to the CV subgroup, in order to achieve an optimal EtCO2 range. Additionally, the EtCO2 and the PaCO2 (P=0.001) were higher in the PV subgroup. PaCO2 values were higher, but considered acceptable, nevertheless, in terms of permissive hypercapnia. Moderate hypercapnia potentiates HPV while also appearing to attenuate the cytokine response (42). Peripheral oxygen saturation at 30 minutes was higher in the CV subgroup (P=0.027). Only two cases of desaturation were registered (one in the CV subgroup and one in the PV subgroup), although, after verification of the DLT position through FOB, both were resolved with recruitment maneuvers without having to increase FiO2. In both cases, patients were not affected by chronic obstructive pulmonary disease (COPD) and had a FEV1 >90%. According to published findings (2), desaturation events during OLV are more likely in patients with better spirometric function prior to surgery. Slinger et al. (43) found that the lower the preoperative FEV1 was, the better oxygenation was during OLV. This paradoxical relationship may be explained by several mechanisms. A “dependent-lung effect”: the trapping of air in the ventilated lung can generate auto-PEEP during OLV decreasing the onset of atelectasis and improving oxygenation. A “non-dependent lung effect “: in COPD patients the non-ventilated lung may collapse more slowly, preserving oxygenation longer. A third mechanism is a redistribution of perfusion: patients with obstructive disease could be chronically subject to a degree of HPV and may have a different redistribution of the perfusion between the lungs during OLV (43).
One patient in the CV subgroup required a change in the ventilation mode from volume-controlled ventilation to pressure-controlled ventilation due to a PIP value >35 cmH2O, with a setting to maintain the same VT. After the ventilation mode was changed, the mean P peak value was reduced to below 30 cmH2O, and the patient required no further assistance.
In the present study, systemic inflammation through blood sample analysis was not explored; this proved to be a potential limit of the study because blood tests could have been useful in determining the attribution of cytokine response in surgical trauma, lung cancer and other comorbidities. Moreover, there was no follow-up for patients after being discharged from the hospital which would have provided valuable insight into the long-term outcome. Finally, the non-randomized design of the study represented a further limit.
In conclusion, the present study showed no localized inflammatory cytokine response and no differences between different ventilatory strategies (regardless of whether a smaller or larger VT was employed) with a standardized application of PEEP and a short duration of OLV. The application of PEEP and short-term OLV could play a key role in preventing the development of VILI and postoperative respiratory complications.
Acknowledgements
Funding: Prof. M Simmaco was responsible for research funding which was acquired from Sant’Andrea Hospital as part of the collaboration agreement signed on 12/21/2012 (Resolution AOSA 753).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Bioethics Committee of Sapienza University of Rome (No. 3722_2015), and informed consent was obtained from each patient in writing prior to enrolment.
References
- Yin K, Gribbin E, Emanuel S, et al. Histochemical alterations in one lung ventilation. J Surg Res 2007;137:16-20. [Crossref] [PubMed]
- Karzai W, Schwarzkopf K. Hypoxemia during One-lung Ventilation Prediction, Prevention, and Treatment. Anesthesiology 2009;110:1402-11. [Crossref] [PubMed]
- Sommer N, Dietrich A, Schermuly RT, et al. Regulation of hypoxic pulmonary vasoconstriction: basic mechanisms. Eur Respir J 2008;32:1639-51. [Crossref] [PubMed]
- Carden DL, Granger DN. Pathophysiology of ischaemia reperfusion injury. J Pathol 2000;190:255-66. [Crossref] [PubMed]
- de la Gala F, Piñeiro P, Garutti I, et al. Systemic and alveolar inflammatory response in the dependent and nondependent lung in patients undergoing lung resection surgery: A prospective observational study. Eur J Anaesthesiol 2015;32:872-80. [PubMed]
- Sugasawa Y, Yamaguchi K, Kumakura S, et al. The effect of one-lung ventilation upon pulmonary inflammatory responses during lung resection. J Anesth 2011;25:170-7. [Crossref] [PubMed]
- Lohser J, Slinger P. Lung Injury After One-Lung Ventilation: A Review of the Pathophysiologic Mechanisms Affecting the Ventilated and the Collapsed Lung. Anesth Analg 2015;121:302-18. [Crossref] [PubMed]
- Licker M, de Perrot M. A risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg 2003;97:1558-65. [Crossref] [PubMed]
- Lytle FT, Brown DR. Appropriate ventilatory settings for thoracic surgery: intraoperative and postoperative. Semin Cardiothorac Vasc Anesth 2008;12:97-108. [Crossref] [PubMed]
- Della Rocca G, Coccia C. Ventilatory management of one-lung ventilation. Minerva Anestesiol 2011;77:534-6. [PubMed]
- Olivant Fisher A, Husain K, Wolfson MR, et al. Hyperoxia during one lung ventilation: inflammatory and oxidative responses. Pediatr Pulmonol 2012;47:979-86. [Crossref] [PubMed]
- Fernández-Pérez ER, Keegan MT, Brown DR, et al. Intraoperative tidal volume as a risk factor for respiratory failure after pneumonectomy. Anesthesiology 2006;105:14-8. [Crossref] [PubMed]
- Yang M, Ahn HJ, Kim K, et al. Does a Protective Ventilation Strategy Reduces the Risk of Pulmonary Complications Following Lung Cancer Surgery?: a Randomized Controlled Trial. Chest 2011;139:530-7. [Crossref] [PubMed]
- Michelet P, D'Journo XB, Roch A, et al. Protective ventilation influences systemic inflammation after esophagectomy: a randomized controlled study. Anesthesiology 2006;105:911-9. [Crossref] [PubMed]
- Theroux MC, Fisher AO, Horner LM, et al. Protective ventilation to reduce inflammatory injury from one lung ventilation in a piglet model. Paediatr Anaesth 2010;20:356-64. [Crossref] [PubMed]
- Breunig A, Gambazzi F, Beck-Schimmer B, et al. Cytokine & chemokine response in the lungs, pleural fluid and serum in thoracic surgery using one-lung ventilation. J Inflamm (Lond) 2011;8:32. [Crossref] [PubMed]
- Schilling T, Kozian A, Huth C, et al. The Pulmonary Immune Effects of Mechanical Ventilation in Patients Undergoing Thoracic Surgery. Anesth Analg 2005;101:957-65. [Crossref] [PubMed]
- Kotani N, Hashimoto H, Sessler DI, et al. Neutrophil number and interleukin-8 and elastase concentrations in bronchoalveolar lavage fluid correlate with decreased arterial oxygenation after cardiopulmonary bypass. Anesth Analg 2000;90:1046-51. [Crossref] [PubMed]
- Frass OM, Buhling F, Tager M, et al. Antioxidant and antiprotease status in peripheral blood and BAL fluid after cardiopulmonary bypass. Chest 2001;120:1599-608. [Crossref] [PubMed]
- Gattinoni L, Protti A, Caironi P, et al. Ventilator-induced lung injury: The anatomical and physiological framework. Crit Care Med 2010;38:S539-S548. [Crossref] [PubMed]
- Dreyfuss D, Saumon G. Ventilator-induced lung injury: Lessons from experimental studies. Am J Respir Crit Care Med 1998;157:294-323. [Crossref] [PubMed]
- Bastin AJ, Sato H, Davidson SJ, et al. Biomarkers of lung injury after one-lung ventilation for lung resection. Respirology 2011;16:138-45. [Crossref] [PubMed]
- Tremblay L, Valenza F, Ribeiro SP, et al. Injurious ventilator strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 1997;99:944-52. [Crossref] [PubMed]
- Kotani N, Takahashi S, Sessler DI, et al. Volatile anesthetics augment expression of proinflammatory cytokines in rat alveolar macrophages during mechanical ventilation. Anesthesiology 1999;91:187-97. [Crossref] [PubMed]
- Chiumello D, Pristine G, Slutsky AS. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med 1999;160:109-16. [Crossref] [PubMed]
- Wrigge H, Zinserling J, Stüber F, et al. Effects of Mechanical Ventilation on Release of Cytokines into Systemic Circulation in Patients with Normal Pulmonary Function. Anesthesiology 2000;93:1413-7. [Crossref] [PubMed]
- Treschan TA, Kaisers W, Schaefer MS, et al. Ventilation with low tidal volumes during upper abdominal surgery does not improve postoperative lung function. Br J Anaesth 2012;109:263-71. [Crossref] [PubMed]
- Levin MA, McCormick PJ, Lin HM, et al. Low intraoperative tidal volume ventilation with minimal PEEP is associated with increased mortality. Br J Anaesth 2014;113:97-108. [Crossref] [PubMed]
- Blank RS, Colquhoun DA, Durieux ME, et al. Management of One-lung Ventilation Impact of Tidal Volume on Complications after Thoracic Surgery. Anesthesiology 2016;124:1286-95. [Crossref] [PubMed]
- Shen Y, Zhong M, Wu W, et al. The impact of tidal volume on pulmonary complications following minimally invasive esophagectomy: A randomized and controlled study. J Thorac Cardiovasc Surg 2013;146:1267-73. [Crossref] [PubMed]
- De Conno E, Steurer MP, Wittlinger M, et al. Anesthetic-induced Improvement of the Inflammatory Response to One-lung Ventilation . Anesthesiology 2009;110:1316-26. [Crossref] [PubMed]
- Tekinbas C, Ulusoy H, Yulug E, et al. One-lung ventilation: for how long? J Thorac Cardiovasc Surg 2007;134:405-10. [Crossref] [PubMed]
- Misthos P, Katsaragakis S, Theodorou D, et al. The degree of oxidative stress is associated with major adverse effects after lung resection: a prospective study. Eur J Cardiothorac Surg 2006;29:591-5. [Crossref] [PubMed]
- Almakadma YS, Riad TH, Ayad II, et al. Duration of one-lung ventilation stage, POSSUM value and the quality of post-operative analgesia significantly affect survival and length of stay on intensive care unit of patients undergoing two-stage esophagectomy. Saudi J Anaesth 2013;7:238-43. [Crossref] [PubMed]
- Schilling T, Kozian A, Senturk M, et al. Effects of Volatile and Intravenous Anesthesia on the Alveolar and Systemic Inflammatory Response in Thoracic Surgical Patients. Anesthesiology 2011;115:65-74. [Crossref] [PubMed]
- Mahmoud K, Ammar A. Immunomodulatory Effects of Anesthetics during Thoracic Surgery. Anesthesiol Res Pract 2011;2011:317410. [PubMed]
- Schilling T, Kozian A, Kretzschmar M, et al. Effects of propofol and desflurane anaesthesia on the alveolar inflammatory response to one-lung ventilation. Br J Anaesth 2007;99:368-75. [Crossref] [PubMed]
- Dikmen Y, Eminoglu E, Salihoglu Z, et al. Pulmonary mechanics during isoflurane, sevoflurane and desflurane anaesthesia. Anaesthesia 2003;58:745-8. [Crossref] [PubMed]
- Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008;248:189-98. [Crossref] [PubMed]
- Schatz C. Enhanced Recovery in a Minimally Invasive Thoracic Surgery Program. AORN J 2015;102:482-92. [Crossref] [PubMed]
- D'Andrilli A, Ibrahim M, Ciccone AM, et al. Intrapleural intercostal nerveblock associated with mini-thoracotomy improve pain control after major lung resection. Eur J Cardiothorac Surg 2006;29:790-4. [Crossref] [PubMed]
- Balanos GM, Talbot NP, Dorrington KL, et al. Human pulmonary vascular response to 4 h of hypercapnia and hypocapnia measured using Doppler echocardiography. J Appl Physiol 2003;94:1543-51. [Crossref] [PubMed]
- Slinger P, Suissa S, Triolet W. Predicting arterial oxygenation during one lung anesthesia. Can J Anaesth 1992;39:1030-5. [Crossref] [PubMed]


