HIV-negative pulmonary disease caused by nontuberculous mycobacteria in Southern Brazil: clinical and microbiological characterization
Introduction
Interest in nontuberculous mycobacteria (NTM) is the result of two increasing trends, the infection with NTM in patients with AIDS (acquired immunodeficiency syndrome) and recognition that pulmonary disease due to NTM has increased greatly among those people not infected with human immunodeficiency virus (HIV) (1). Among the potentially pathogenic NTM and most frequently isolated in the clinical practice are M. avium, M. intracellulare, M. kansasii, M. chelonae, M. abscessus, M. fortuitum, and M. peregrinum. Several NTM can be isolated from the culture of clinical specimens and do not necessarily mean that they are the cause of the pulmonary pathology under investigation (2).
NTM present variable pathogenic feature and many species do not cause disease while others are highly pathogenic and can even result in death (3). More recently, the presence of disease caused by NTM has frequently been described in people not infected with HIV and with structural damaged due to chronic pulmonary disease (3,4).
M. avium and M. intracellulare, referred previously as M. avium complex (MAC) are the most common NTM associated with human diseases and the differentiation between these two species is not always performed. In the vast majority (>95%) of patients with AIDS and MAC the infection is due to M. avium and occurs when the CD4 count is lower than 50 cells/mm3 (5). In non-HIV population M. intracellulare increases in frequency and exhibits a more severe presentation and a worse prognosis than patients with M. avium lung disease. Patients with pulmonary disease by M. intracellulare tend to present more severe manifestations due to disease progression and less treatment response (6,7). It has been discussed if some more stringent criteria should be used for species with low clinical relevance, and less stringent criteria applied for species of high clinical relevance in that geographic area (8).
Epidemiological data NTM disease in Brazil is scarce. Brazil is a continental country and NTM isolation rates are quite different among different regions, but there is a predominance of MAC, M. kansasii and M. fortuitum causing active NTM disease. Brazil is a high tuberculosis (TB) burden country and had for many years overshadowed the role of NTM in human disease (9,10).
The aim of this study was to identify NTM species isolated in the context of infection/colonization versus active pulmonary disease and to evaluate the main factors associated with NTM pulmonary disease and their outcomes in a Brazilian tertiary hospital.
Methods
This is a descriptive cross-sectional study that included patients from the Tisiology clinic at Ribeirão Preto (Southern Brazil) who had at least one NTM isolated in respiratory specimens (sputum, bronchoalveolar lavage, induced sputum, early-morning gastric lavage), from January 2011 to December 2014. Patients whose isolates were not identified to the level of mycobacterial species were excluded.
NTM cases were defined based on the American Thoracic Society (ATS) criteria which have issued diagnostic criteria to aid diagnosis of pulmonary NTM disease cases and distinguish them from simple colonization (11). All cases with NTM isolation were evaluated retrospectively.
Processing of samples
NTM mycobacteria culture was performed in liquid medium, in the mycobacteria growth indicator tube (MGIT) automated system (Becton Dickinson Loveton Circle Sparks, Sparks Glencoe, MD, USA). NTM identification was made by immunochromatographic test (TB TEST BIOEASY® Ag MPT64, Belo Horizonte, Brazil) using the anti-MPT64 monoclonal antibody. All the NTM isolates were then forwarded to the State Central Laboratory for species determination by the phenotypic and molecular typing technique. In the phenotypic identification, the strains were subjected to macroscopic screening (morphology and pigmentation of colonies) and microscopic examination (morphology of the acid-fast bacilli and cord factor observation) for presumptive identification. Strains with pigmented or smooth acromogenic colonies that did not present rope formation in the microscopic test received a presumptive classification of NTM and were subjected to phenotypic tests of growth time and temperature analysis.
The polymerase chain reaction (PCR) amplification method and subsequent restriction analysis of a 441 bp fragment of the hsp65 gene (hsp65 PRA) was used for the molecular identification of the species. The DNA was extracted and amplified from the bacterial mass after ten minutes of boiling, the amplified fragments were separately digested with the BstEII and HaeIII restriction enzymes and their products separated by electrophoretic run on 3% agarose gel. The restriction pattern was analyzed to obtain the species (10).
All the records from patients with NTM identified till the species level were reviewed and a form was completed including demographic, clinical, radiological and microbiological information, the definitive diagnosis, treatment and outcome after 12 months of starting treatment for NTM. The outcomes were assessed 12 months after the first isolation of a NTM and described as: (I) cure (clinical response plus radiological improvement and microbiological negative results); (II) improved after TB treatment (clinical, radiological and microbiological response after TB treatment with rifampicin, isoniazid, pyrazinamide and ethambutol); (III) improved without NTM treatment; (IV) not cure (anything different from what was defined as cure); (V) death due to others causes or before NTM confirmed diagnosis; and (VI) lost follow-up.
Associations involving the qualitative variables of the study were made using the chi-square test or Fisher’s exact test and for the comparative assessment of the age, Student’s t-test was used. All analyzes were performed using the SAS 9.0 software. For all comparisons a 5% significance level was considered.
The IRB clearance was obtained from Research Ethics Committee of the Clinics Hospital at Ribeirão Preto Medical School, under authorization number 9586/2011.
Results
From 2011 to 2014 the mycobacterial laboratory performed 14,394 cultures for mycobacteria in respiratory specimens. Of these, 590 (4.10%) showed growth of NTM and 2,087 (14,5%) detected M. tuberculosis. There were 305 (51.7%) NTM isolates characterized till the species level and which were included in the study, representing 290 patients. Of these, 79 (27.2%) patients with HIV infection were excluded from the analysis. The ATS criteria (2007) were applied to the 211 (72.8%) remaining, with 49 (23.2%) fulfilling the criteria for disease caused by NTM and 162 (76.8%) being characterized only as colonized patients (without NTM criteria for disease). Patients’ description based on age, gender, respiratory symptoms, presence of image suggestive of NTM involvement, previous treatment for TB, NTM treatment and death are shown in Table 1.
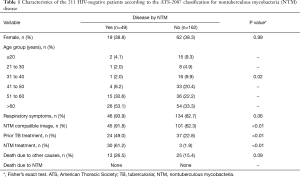
Full table
The NTM species most often isolated in this group was M. intracellulare in 59.1% (29 isolates), followed by M. avium in 14.3% (7), M. abscessus 12.2% (6), M. gordonae 6.1% (3), M. fortuitum 4.1% (2) and other NTM 4.1% (2). The smear microscopy was positive in 18.4% of patients in this group (Table 2).
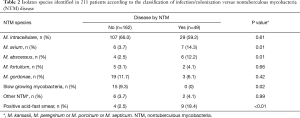
Full table
Among the patients without confirmed NTM disease (162 colonized individuals) the most frequent species identified were: M. intracellulare (66.1%), M. gordonae (11.7%), slow growing NTM (9.3%), M. avium (3.7%) and M. fortuitum (3.1%).
Outcomes for cases with confirmed disease by NTM
Among 49 confirmed NTM cases there were 30 (61.2%) who started specific treatment and 19 (38.8%) who had not been treated. Patients’ records were reviewed 12 months after treatment initiation for the first group and 12 months after disease confirmation for the other 19 patients without treatment. There were 17 (56.7%) out of 30 treated patients who had been cured; 7 (23.3%) completed the treatment and had no improvement; 4 patients (13.3%) lost follow-up; and 2 (6.7%) died from other causes (cancer).
Among 19 NTM cases not treated the reasons to not start treatment were: follow-up lost, death due to other causes before the results of NTM cultures were available; misdiagnose and treatment for TB after positive acid-fast smear results; other concomitant priority diagnosis (i.e., lung, rectum or hematologic cancer, pulmonary paracoccidioidomycosis or aspergillosis). The outcomes observed in this group after the initial diagnosis was: 8 (42.1%) patients improved [2 (25%) after TB treatment and 6 (75%) without specific treatment]; 5 (26.3%) patients died [3 (60%) before the NTM culture results were available and 2 (40%) due to cancer]; 6 (31.6%) patients lost follow-up (Table 3).
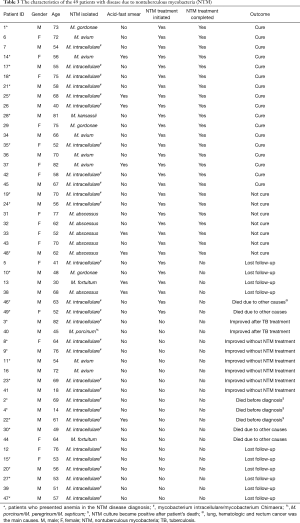
Full table
Patients who had clinical response, radiological improvement and negative culture after 12 months of follow-up were usually infected with M. avium, M. kansasii and M. intracellulare. None of the patients with M. abscessus had complete regression of the clinical findings or negative culture results after one year under treatment (Table 3).
By classifying the NTM disease for non-HIV patients with pulmonary disease according to the McShane and Glassroth (2015) proposal (2), there were 12 patients with nodular pattern; 11 with bronchiectasis plus nodular pattern; 7 patients with a mixed pattern (nodules, bronchiectasis and cavitation); 2 patients with TB like pattern; and no one with hypersensitivity pneumonitis. Other findings in the thorax imaging were described in 17 patients, like consolidation, atelectasis, ground-glass opacification, pleural effusion, pleural thickening and single calcified nodule.
In this study, 27 patients (55%) presented anemia when they were diagnosed with NTM pulmonary disease and 3 (42.8%) had no improvement after 12 months of treatment (Table 3).
In Table 4 we describe data about pulmonary NTM studies in Brazil including the data we got for patients with NTM disease and those with only colonization (12-19), based on the ATS-2007 criteria (11).
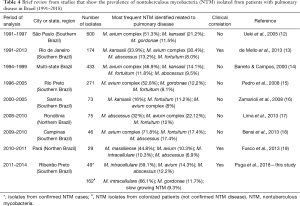
Full table
Discussion
For reasons that are not fully understood, some pathogenic NTM tend to cluster in specific geographic regions. MAC members are the most frequently species isolated NTM worldwide. A study with species identification for 20,182 patients, from 62 laboratories in 30 countries across 6 continents showed 91 different NTM species isolated. MAC predominated in most countries, followed by M. gordonae and M. xenopi. Important differences in geographical distribution of MAC species as well as M. xenopi, M. kansasii and rapid-growing mycobacteria were observed. M. avium was the most frequent isolate in North and South America, while M. intracellulare was more frequent in Australia with 80% of the cultures having MAC growth and 77.5% in South Africa (20).
In Brazil, NTM is not a disease for compulsory notification so there are no official records of prevalence. Data from Rio de Janeiro with 5,448 patients with positive smear microscopy treated in the outpatient clinic, between 1995 and 1996, showed a prevalence of 5.83/1,000 people NTM disease cases (21). From 1991 to 1997 a study with 1,892 NTM isolates from 1,248 patients in the state of São Paulo, showed that MAC and M. kansasii were the most frequently species identified (12). Nunes-Costa et al. (2016) reviewing the literature about NTM infections in Brazil and Portugal found that MAC was the most reported NTM in Brazilian studies (48%), followed by M. Kansasii (17%), M. fortuitum (7%), M. gordonae (6%), M. abscessus (5%) and M. chelonae (3%), quite similar to what is observed in Portugal (9). The findings of our study showed that M. intracellulare is the most frequent NTM isolated in pulmonary specimens from patients with confirmed NTM disease in Southern Brazil, followed by M. avium and M. abscessus. As a continental country Brazil has important differences according to the geographic region, as showed in Table 4.
In Queensland, Australia, the incidence of pulmonary disease by NTM increased from 2.2 to 3.2 per 100,000 people between 1999 and 2005. During this period, the affected population changed from middle-aged male smokers to non-smoking older adult women (3,22). A study in India analyzed 263 cultures with growth of NTM, with 79.4% of these isolated from respiratory specimens from 2013 to 2015. The most common species were M. abscessus, M. fortuitum, M. intracellulare, M. chelonae and M. avium (23). Most of the prevalence studies did not evaluate the correlation between the NTM species, disease definition and the outcomes.
The prevalence and clinical significance of M. avium and M. intracellulare were analyzed in 7,472 patients, from 1999 to 2003, which had been treated at the hospital of the University of Texas. Of the 7,472 patients, 133 initially had at least one MAC isolate. M. avium was isolated in 62 (0.83%) out of 7,472 patients and M. intracellulare in 65 (0.87%). Clinically, only 10 (16.1%) out of 62 patients with M. avium showed evidence of infection. In contrast, 41 (63.1%) of 65 patients with M. intracellulare showed active disease, a much higher level than those with M. avium (P<0.001). There was a greater prevalence in women aged over 60 years. It is suggested that among patients not infected with HIV, M. intracellulare is more pathogenic and has a tendency to infect post-menopausal women (5). Koh et al., (2012) compared patients with M. avium and M. intracellulare lung disease. The patients with M. intracellulare disease were more likely to be older age, had a lower body index mass, more respiratory symptoms and history of previous treatment for TB. Fibrocavitary form of the disease, smear-positive sputum and an unfavorable microbiologic response after combination antibiotic treatment were also more present in M. intracellulare disease (6).
In this study one quarter of the patients presented criteria to define NTM disease. The majority were male (61.2%), over the age of 51 years who a previous history of previous treatment for TB (53.3%) and with structural and fibrotic damage in the lungs (sequelae) in the chest imaging (X-ray/computed tomography). In the women, less than half (42.1%) had previously been treated for TB and chest imaging (X-ray/computed tomography) mostly showed a nodular pattern with or without bronchiectasis. Previous TB treatment and the consequent structural damage seems to be one of the factors associated with NTM disease in high-burden TB countries, like Brazil and those undeveloped countries where smoking is endemic.
Most of the patients (76.8%) in this study were considered colonized/infected. A study performed in Denmark included more than 1,200 adults and 2,666 samples positive for NTM. Strict criteria were used to categorize these isolates and 26% were classified as being responsible for disease caused by NTM, 19% as possibly causing disease and 55% as colonization. Among those patients characterized as having “disease caused by NTM” the risk of progressing to death was 1.33 higher than for those merely colonized (3).
In a retrospective review of cases, performed in Canada, which included adult patients with pulmonary disease who were treated for MAC and monitored for at least 6 months to evaluate the clinical and microbiological results, 107 patients were included (79% female, mean age 67 years). The smear microscopy was positive in half (54%) of the patients (24).
In this study, smear positive microscopy in the sputum was 7 times higher in NTM cases of disease than in the colonized patients (18.4% versus 2.5%; P<0.01), and most of the pulmonary colonization was related M. intracellulare, and not with M. avium or M. abscessus which were more related to NTM pulmonary disease.
McShane and Glassroth (2015) describe an association between anemia and poor prognosis of cases of disease caused by NTM which was observed also in our findings, but in less extent (2).
Different species distribution may partially determine the frequency and the type of pulmonary manifestations for NTM disease in each geographical location. In Southern Brazil there was a predominance of disease caused by NTM in elderly men, with previous TB and mainly with MAC (59.1% M. intracellulare and 14.3% M. avium) followed by M. abscessus (12.2%).
Microbiology is one of the pillars for the diagnosis of the disease caused by NTM and facilitates the definitive diagnosis, contributes to decision making about the need for and choice of treatment (3).
For its retrospective nature, this study has some intrinsic limitations like the absence of species identification in all the isolates during the study period. All the 285 isolates which were not characterized to the mycobacterial species level were unique isolates where there was not a strong suspicion of NTM disease. Another limitation is related to the reliability of some information that could not be checked, so the available data was restricted to what was obtained from the patients’ medical records review.
Conclusions
Patients with more than 50 years old with nodular plus bronchiectasis radiographic pattern, previous TB treatment and a M. intracellulare isolated from respiratory specimens is the prototype of NTM disease in Southern Brazil. Persistence of positive culture without clinical and radiological improvement is the rule when M. abscessus the NTM-disease cause. The same was observed with few isolates of M. intracellulare with extended resistance. The presence of a positive acid-fast smear in the respiratory specimen is a strong predictor of NTM active disease.
Acknowledgements
We want to thank Fundação de Apoio ao Ensino, Pesquisa e Assistência (FAEPA) do Hospital das Clínicas da FMRP-USP.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The IRB clearance was obtained from Research Ethics Committee of the Clinics Hospital at Ribeirão Preto Medical School, under authorization number 9586/2011.
References
- Griffith DE. Nontuberculous mycobacterial lung disease. Curr Opin Infect Dis 2010;23:185-90. [Crossref] [PubMed]
- McShane PJ, Glassroth J. Pulmonary disease due to nontuberculous mycobacteria, current state and new insights. Chest 2015;148:1517-27. [Crossref] [PubMed]
- Weiss CH, Glassroth J. Pulmonary disease caused by nontuberculous mycobacteria. Expert Rev Respir Med 2012;6:597-612. [Crossref] [PubMed]
- Sexton P, Harrison AC. Susceptibility to nontuberculous mycobacterial lung disease. Eur Respir J 2008;31:1322-33. [Crossref] [PubMed]
- Han XY, Tarrand JJ, Infante R, et al. Clinical significance and epidemiologic analyses of Mycobacterium avium and Mycobacterium intracellulare among patients without Aids. J Clin Microbiol 2005;43:4407-12. [Crossref] [PubMed]
- Koh WJ, Jeong BH, Jeon K, et al. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M. avium complex lung disease. Chest 2012;142:1482-8. [Crossref] [PubMed]
- Kwon YS, Koh WJ. Diagnosis and treatment of nontuberculous mycobacterial lung disease, Korea. J Korean Med Sci 2016;31:649-59. [Crossref] [PubMed]
- Jankovic M, Sabol I, Zmak L, et al. Microbiological criteria in non-tuberculous mycobacteria pulmonary disease: a tool for diagnosis and epidemiology. Int J Tuberc Lung Dis 2016;20:934-40. [Crossref] [PubMed]
- Nunes-Costa D, Alarico S, Dalcolmo MP, et al. The looming tide of nontuberculous mycobacterial infections in Portugal and Brazil. Tuberculosis (Edinb) 2016;96:107-19. [Crossref] [PubMed]
- Chimara E, Ferrazoli L, Ueki SY, et al. Reliable identification of mycobacterial species by PCR-restriction enzyme analysis (PRA)-hsp65 in a reference laboratory and elaboration of a sequence-based extended algorithm of PRA-hsp65 patterns. BMC Microbiol 2008;8:48. [Crossref] [PubMed]
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367-416. [Crossref] [PubMed]
- Ueki SY, Martins MC, Telles MA, et al. Nontuberculous mycobacteria: species diversity in São Paulo state, Brazil. J Bras Patol Med Lab 2005;41:1-8. [Crossref]
- de Mello KG, Mello FC, Borga L, et al. Clinical and therapeutic features of pulmonary nontuberculous mycobacterial disease, Brazil, 1993-2011. Emerg Infect Dis 2013;19:393-9. [PubMed]
- Barreto AM, Campos CE. Micobactérias Não Tuberculosas no Brasil. Bol Pneumol Sanit 2000;8:23-32.
- Pedro Hda S, Pereira MI, Goloni Mdo R, et al. Nontuberculous mycobacteria isolated in São José do Rio Preto, Brazil between 1996 and 2005. J Bras Pneumol 2008;34:950-5. [Crossref] [PubMed]
- Zamarioli LA, Coelho AG, Pereira CM, et al. Descriptive study of the frequency of nontuberculous mycobacteria in the Baixada Santista of the state of São Paulo, Brazil. J Bras Pneumol 2008;34:590-4. [Crossref] [PubMed]
- Lima CA, Gomes HM, Oelemann MA, et al. Nontuberculous mycobacteria in respiratory samples from patients with pulmonary tuberculosis in the state of Rondônia, Brazil. Mem Inst Oswaldo Cruz 2013;108:457-62. [Crossref] [PubMed]
- Bensi EP, Panunto PC, Ramos Mde C. Incidence of tuberculous and nontuberculous mycobacteria, differentiated by multiplex PCR, in clinical specimens of a large general hospital. Clinics (Sao Paulo) 2013;68:179-84. [Crossref] [PubMed]
- Fusco da Costa AR, Falkinham JO 3rd, Lopes ML, et al. Occurrence of nontuberculous mycobacterial pulmonary infection in an endemic area of tuberculosis. PLoS Negl Trop Dis 2013;7:e2340. [Crossref] [PubMed]
- Hoefsloot W, van Ingen J, Andrejak C, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 2013;42:1604-13. [Crossref] [PubMed]
- Leão SC, Grinbaum RS. Micobactérias não tuberculosas. In: Veronesi R, Focaccia R. editors. Tratado de infectologia. São Paulo: Atheneu, 2009:1335-43.
- Thomson RM. NTM working group at Queensland TB Control Centre and Queensland Mycobacterial Reference Laboratory. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis 2010;16:1576-83. [Crossref] [PubMed]
- Umrao J, Singh D, Zia A, et al. Prevalence and species spectrum of both pulmonary and extrapulmonary nontuberculous mycobacteria isolates at a tertiary care center. Int J Mycobacteriol 2016;5:288-93. [Crossref] [PubMed]
- Marras TK, Mendelson D, Marchand-Austin A, et al. Pulmonary nontuberculous mycobacterial disease, Ontario, Canada,1998-2010. Emerg Infect Dis 2013;19:1889-91. [Crossref] [PubMed]


