Antiplatelet and anticoagulant for prevention of reocclusion in patients with atrial fibrillation undergoing endovascular treatment for low extremity ischemia
Introduction
Endovascular therapy with stent has been widely used in the treatment for patient with lower extremity ischemia. However, reocclusion occurs in patients undergoing stent implantation in endovascular treatment for low extremity ischemia. Intimal hyperplasia and stent thrombi are two main causes of the reocclusion after the stent implantation (1). Studies have shown that platelet deposits on the thrombogenic surface liberates platelet-derived growth factor, leading to vascular smooth muscle cell proliferation and migration and enhances neointimal formation (2,3). And the active platelet also plays an important role in the formation of stent thrombi.
Accordingly, dual-antiplatelet therapy (DAPT) with aspirin and clopidogrel are mostly prescribed after endovascular intervention for the lower extremity ischemia (4-6). Aspirin inhibits thromboxane A2 (TXA2)-dependent platelet function via inactivating permanently the cyclooxygenase activity to reduce the biosynthesis of pituitary growth hormone (PGH)2 which is the immediate precursor of TXA2 (7). Clopidogrel are ADP receptors antagonists which irreversibly block the ADP receptor on platelets thus leading to the inhibition of ADP-induced platelet aggregation (8). The combination of aspirin and clopidogrel blocks complementary pathways of platelet aggregation and decreases restenosis or stent thrombosis in the endovascular interventions (6,8).
However, clinical studies have reported that approximately 0.4% patients with atrial fibrillation (AF) suffer from low extremity ischemia (9). Patients with AF who have additional risk factors for stroke or systemic thromboembolism benefit from anticoagulant therapy (10,11). For this reason, the use of triple therapy (a dual antiplatelet regimen plus anticoagulant) for these patients is expected to become prominent after the endovascular intervention in the lower extremity. However, triple therapy increases the risk of bleeding events and the regimen of mono antiplatelet plus oral anticoagulant has been demonstrated a similar stent thrombi rate but a lower rate of clinically significant bleeding than triple therapy in patient with AF undergoing percutaneous coronary intervention (PCI) (12). Whether the mono antiplatelet plus oral anticoagulant therapy is effective and safe for patients with AF undergoing endovascular intervention for low extremity ischemia remains uncertain and this study retrospectively investigated the outcomes of aspirin plus oral anticoagulant therapy for them post-operatively.
Methods
From March 2014 to July 2016, 32 consecutive symptomatic patients (11 women and 21men; mean age, 77 years; range, 68–84 years) with lower extremity ischemia who also suffer from AF were eligible for endovascular treatment in our center; 12 patients with acute lower extremity ischemia (defined as within 14 days), 17 patients with chronic lower extremity ischemia (over 14 days), and 3 patients with acute on chronic ischemia (chronic lower extremity ischemia with acute deterioration) were included in this study respectively (Table 1). Patients selected in the study had to meet the following criteria: (I) AF suggested by electrocardiogram; (II) major lesions of femopopliteal arteries; (III) at least one distal run-off below the knees after the intervention. While the excluded patients concluded those: (I) with digestive tract hemorrhage or ulcer; (II) undergoing active bleeding during the past 3 months (such as major surgical operation, severe injuries); (III) with thrombophilia; (IV) with stroke history within 6 months; (V) having serious or unmanageable hypertension and contraindications to the use of anticoagulation, contrast media, or thrombolytic agents.
Full table
Our clinical indication for endovascular therapy was moderate to severe claudication or critical limb ischemia. Preprocedural assessment was performed by means of computed tomography angiography or magnetic resonance angiography and reconstructions were obtained to evaluate the site, length, type (thrombus, calcific), proximal occlusion level, and distal runoff vessels of the lesion. The protocol of this study was carried out according to the principles of the Declaration of Helsinki and approved by the medical ethics committee in our Hospital. Written informed consent was obtained from each patient after a detailed explanation of the risks and benefits of the procedure.
All procedures were performed in the angiography unit under local anesthesia. With the patient under local anesthesia, arterial access was performed initially by using a percutaneous retrograde femoral approach in all patients with a 6-F cross sheath (Cook Medical, Bloomington, IN, USA) and heparin (125 IU/kg) was administered through the sheaths to prevent development of pericatheter thrombus. The initial angiography was performed from the sheath and the guide wire (Terumo, Tokyo, Japan) was then crossed through the occluded lesions. Before May 2015, a selected multi-side-hole thrombolytic catheter (Angio Dynamics, NY, USA) was advanced over the guide wire into the site of the target lesion for acute thrombus or acute on chronic according to the length of the occlusion. After May 2015, thrombectomy were performed using 6/8F Straub Rotarex to reduce ischemic symptoms and a few patients who were revealed a residual thrombus by angiography underwent catheter-directed thrombolysis (CDT). Complete details of the urokinase and unfractionated heparin infusion and laboratory monitoring were according to previously studies (13).
Adjunctive endovascular treatments including percutaneous transluminal angioplasty (PTA) and stenting were performed to correct residual stenosis of the lesions after the thrombolytic/thrombectomy procedure or to correct native lesions for chronic patients. After ballooning (4–6 mm in diameter, 60–200 mm long; Admiral Xtreme, Invatec, Italy), self-expandable bare stents (5–7 mm in diameter, 60–200 mm long; EV3, Plymouth, MN, USA; Complete SE, Meditronic, MN, USA; or Smart, Cordis, NJ, USA) were used to correct underlying lesions with post-dilation. A completion angiography was performed to assess the status of patency of femopopliteal artery.
Technical success was defined as restored artery patency with a residual diameter stenosis of <30%. Clinical success was defined as a subjective perception of improved walking distance that resulted in an improved classification based on the Rutherford category.
Peri-procedural complications included major bleeding (intracranial bleeding, bleeding that resulted in death, or bleeding that required transfusion, surgery, or cessation of thrombolytic therapy), minor bleeding (defined as less severe bleeding managed by local compression, increasing vascular sheath size, or decreasing dose of thrombolysis, anticoagulation, or antiplatelet drug), distal runoff embolization, and acute renal failure etc. Postoperative complications include gastrointestinal bleeding, intracranial bleeding etc.
Patients were discharged on an oral regimen of aspirin (100 mg/d), and warfarin [with dose adjustment to achieve a target international normalized ratio (INR) of 2.0 to 3.0] or rivaroxaban (10 mg/d). Clinical examination, the ankle-brachial index (ABI) and duplex ultrasound imaging were performed before discharge, at 1, 3, and 6 months after discharge, and every 6 months thereafter. Restenosis was defined as >2.4 of peak systolic velocity ratio by duplex imaging. Computed tomography angiography was performed only in cases where recurrent stenosis was >70% diameter reduction as measured on ultrasonography scans.
Statistical analysis was performed with SPSS software version 18 (SPSS Inc., Chicago, IL, USA). The cumulative primary patency rates were assessed with Kaplan-Meier curve. The statistical differences in ABI values and Rutherford classification before and after intervention were analyzed with the paired nonparametric test. P < 0.05 was considered statistically significant.
Results
A total of 32 patients with femopopliteal lesions underwent endovascular therapy in our institution and 5 (15.6%) patients combined with of iliac lesions. Severity of ischemia, as defined according to the Rutherford category included: class III, severe claudication in 6 patients; class IV ischemic rest pain in 19 patients; and class V minor tissue loss in 7 patients. Patient comorbidities included hypertension in 32 patients, coronary artery disease in 12 patients, diabetes mellitus in 10 patients, chronic renal insufficiency in 3 patients, cerebral infarction in 12 patients, and smoking in 6 patients (Table 1).
Complete reconstruction of occluded femopopliteal arteries with unimpeded blood flow to legs were successfully obtained in 32 patients. Concomitant unilateral iliac artery was recanalized simultaneously in 5 patients, and among them, directed PTA and stenting in 3 patients, and CDT (or thrombectomy) with stenting angioplasty in 2 patients. There were mean 1.5 infrapopliteal outflow vessels before operation (no vessel in 4 patients, 1 vessel in 15 patients, 2 vessels in 6 patients, 3 vessels in 7 patients). During operation, ballooning angioplasty was achieved in 14 patients, stenting angioplasty in 4 patients, and thrombolysis in 1 patient. After operation, there were mean 2.1 infrapopliteal outflow vessels (1 vessel in 6 patients, 2 vessels in 17 patients, 3 vessels in 9 patients).
Among all superficial femoral lesions, mechanical thrombectomy for 6 patients with acute ischemic history, and CDT for 10 patients (6 patients with acute ischemia, 1 patient with chronic ischemia, 3 chronic patients with acute deterioration). For the chronic patients with 2 months’ ischemia, the guide wire was crossed through the occluded thrombus easily, and a 20-cm-length thrombolytic catheter was advanced into the thrombus. After 36-h thrombolysis, the lesion length decreased from 140 to 80 mm. Among all 10 patents with CDT, the thrombolytic procedure lasted an average of 42.5 h (range, 37–54 h) with a mean urokinase dose of 1,606,500 IU (range, 1,198,800–1,814,400 IU) (Table 2). Among 16 chronic ischemic patients with directed PTA and stenting, subintima angioplasty was achieved in one patient, and retrograde peroneal or post tibial access was chosen when occlusive lesion could not be revascularized through an antegrade approach for another two patients.
Full table
Perioperative complications include: one patients experienced distal embolization, and the thrombus was absorbed with guiding catheter. Hematomata at puncture sites in two patients (2/32) were compressed with pressure bandages during post operation and no pseudoaneurysm occurred. No episodes of major bleeding and only one patient showed positive fecal occult blood tests during the follow-up. The level of hemoglobin hasn’t significantly changed.
Postoperative medication includes aspirin plus rivaroxaban therapy for 20 patients and 12 patients receive aspirin plus warfarin instead. After a mean follow-up of 19.5 months (range, 6–34 months), all 32 patients showed significant improvements in symptoms and 4 patients improved completely; 16 improved to Rutherford category 1 (mild claudication), and 11 were in Rutherford category 2 (moderate claudication), and 1 were in Rutherford category 3 (severe claudication) which corresponded to an improvement by 2 categories in 13 patients, by 3 categories in 14 patients, and by 4 categories in 5 patients. The difference in the distributions of Rutherford category between baseline and follow-up was statistically significant (P<0.001). As expected, the mean ABI increased from 0.43±0.21 preoperatively to 0.81±0.16 postoperatively (P<0.01) (Table 2).
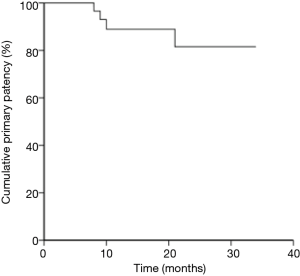
The patency rates were obtained according to the Kaplan-Meier method, and the primary patency rates were 88.9% at 12 months, and 81.5% at 24 months (Figure 1). In-stent restenosis (defined as >70% recurrent luminal narrowing) of the femopopliteal artery was diagnosed in four patients on the basis of duplex ultrasound. Two (7.7%) patients underwent repeat endovascular treatment, while another two (7.7%) patients had no lifestyle-limiting symptoms and refused re-intervention.
Discussion
With the evolution of pharmacological strategies and techniques in endovascular intervention, the patency of patients undergoing endovascular therapy have been significantly improved. However, reocclusion still remains a complication of stent implantation and main causes of the reocclusion after the stent implantation are intimal hyperplasia and stent thrombi (1). Platelet aggregation and attraction of inflammatory cells play an important role in the formation of reocclusion during endovascular interventions. Platelet aggregation at the site of the damaged arterial wall results in the organization of the thrombus through activating platelet-derived growth factor receptor (PDGFR), particularly through PI3K and PLC (2,14). Attraction of inflammatory cells after artery wall injury plays a central role in restenosis after stenting and drives fibroblast growth and smooth muscle cell hyperplasia (1). Accordingly, in clinical practice, DAPT has been widely prescribed as post-operation therapy in the prevention for the reocclusion in these patients (15).
However, approximately 0.4% patients with AF have been reported suffer from low extremity ischemia and anticoagulant therapy has been recommended for them. The triple therapy (dual antiplatelet therapy with a P2Y12 inhibitor and aspirin plus anticoagulant) is expected to be a more prominent for patients with AF undergoing endovascular treatment for low extremity ischemia. However, studies have indicated that triple therapy leads to an increased risk for bleeding events in patients with AF undergoing PCI (12). Paikin et al. has reported that triple therapy may result in excessive major bleeding with rates of 2.2% within the first month and 4 to 12% within the first year of treatment (16). Gibson et al. has reported the different drug therapy in those with AF undergoing PCI (12). Patients in group 1 received mono antiplatelet plus anticoagulant therapy while patients in group 2 received triple therapy. The patients in two group indicated similar efficacy to prevent stent thrombosis (0.8% in group 1 and 0.9% in group 2) and similar composite event rates (6.5% in group 1 and 5.6% in group 2). But the bleeding rate is significantly lower in group 1 (16.8%)than that in group 2 (18.0%).
In our study, only one patient showed positive fecal occult blood tests during follow-up and the rates of bleeding events in our study (3.1%) was also significantly lower when compared to other studies, which indicated the superiority of the mono antiplatelet plus oral anticoagulant therapy for these patients.
In this study, a postoperative therapy which is composed of a mono antiplatelet regimen plus anticoagulant was prescribed to reduce the reocclusion in our patients and long-term results are encouraging. The primary rates were 88.9% and 81.5% at 12 and 24 months respectively, which is higher than the reported patency of approximately 70% at 12 months and 50% at 24 months (17). In our study, 20 patients receive aspirin plus rivaroxaban post-operation and 12 patients receive aspirin plus warfarin instead. Conventional oral anticoagulant includes coagulation factor X (FX) inhibitors (rivaroxaban) and coagulation factor II (FII) inhibitors (dabigatran). The mechanisms by which FX preventing restenosis may include: (I) FX is vital to the pathway in restenosis by influencing coagulation cascade. FXa, a serine protease plays a central role in the coagulation cascade linking the extrinsic and intrinsic pathway which converts prothrombin to thrombin. FXa associated with coagulation factor Va (FVa) and phospholipids forms the prothrombinase complex and activates prothrombin to thrombin. (II) FXa plays an important role in the pro-inflammatory responses through protease activated receptors (PARs) in many cell types such as endothelial cells, platelets, fibroblasts, and endothelial cells (18,19). (III) Functioning through the PARs, FX influences intimal hyperplasia which is one of the main causes of the reocclusion. FX and FXa and thrombin, potent mitogens of both cultured fibroblasts and smooth muscle cells in vitro may be responsible for the hyperplasia of smooth muscle cells and intimal hyperplasia in the pathway of stent stenosis (20). By inhibiting the activation of FXa, Rivaroxaban attenuate restenosis in our study. Other studies have also shown that rivaroxaban reduces restenosis after balloon angioplasty of atherosclerotic femoral arteries in rabbits (21). However, warfarin may mainly exert its effect by influencing the coagulation cascade with more limited effects than rivaroxaban, that may account for why warfarin has not been a routine for patients after stent implantation previously.
The mono antiplatelet plus anticoagulant therapy appears feasible for patients with AF undergoing endovascular treatment for low extremity ischemia with favorable midterm patency. Apart from the use of antiplatelet plus anticoagulation therapy, there are several other possible factors contributing this result: (I) long-term benefits may be the result of the opening of dormant infrapopliteal arteries. In our study, patients with embolism in lower extremity may show better condition of runoff vessels than those with atherosclerotic occlusive ischemia. (II) In addition, thrombus was successfully attenuated with CDT or mechanical thrombectomy. And more, thrombolysis is not only feasible for acute patients, but also for acute on chronic cases (13).
Limitations of this study includes the insufficient follow-up time and small sample size. Furthermore, this is not a randomized study including a reference group. In addition, patients with lower extremity ischemic all suffer from AF in our study, the efficacy of the aspirin plus rivaroxaban therapy for patients with lower extremity ischemic because of arteriosclerosis remains uncertain. Further multicenter clinical trials are expected to prove the efficacy and safety of the mono antiplatelet plus oral anticoagulant therapy for patients undergoing endovascular treatment for lower extremity arteriosclerotic ischemia.
Conclusions
The mono antiplatelet (aspirin) plus anticoagulation therapy offers a safe and effective alternative to the triple therapy for prevention of reocclusion in patients with AF undergoing endovascular treatment for lower extremity ischemic.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (8150039).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics committee of Changhai Hospital (CHEC-2016-67) and written informed consent was obtained from all patients.
References
- Byrne RA, Joner M, Kastrati A, et al. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur Heart J 2015;36:3320-31. [Crossref] [PubMed]
- Caglayan E, Vantler M, Leppänen O, et al. Disruption of platelet-derived growth factor-dependent phosphatidylinositol 3-kinase and phospholipase Cγ 1 activity abolishes vascular smooth muscle cell proliferation and migration and attenuates neointima formation in vivo. J Am Coll Cardiol 2011;57:2527-38. [Crossref] [PubMed]
- Verhaeghe R. Prophylactic antiplatelet therapy in peripheral arterial disease. Drugs 1991;42 Suppl 5:51-7. [Crossref] [PubMed]
- Olin JW, White CJ, Armstrong EJ, et al. Peripheral artery disease: evolving role of exercise, medical therapy, and endovascular options. J Am Coll Cardiol 2016;67:1338-57. [Crossref] [PubMed]
- Spiliopoulos S, Katsanos K, Pastromas G, et al. Initial experience with Ticagrelor in patients with critical limb ischemia and high on-clopidogrel platelet reactivity undergoing complex peripheral endovascular procedures. Cardiovasc Intervent Radiol 2014;37:1450-7. [Crossref] [PubMed]
- Pastromas G, Spiliopoulos S, Katsanos K, et al. Clopidogrel responsiveness in patients undergoing peripheral angioplasty. Cardiovasc Intervent Radiol 2013;36:1493-9. [Crossref] [PubMed]
- Patrono C, Coller B, Dalen JE, et al. Platelet-active drugs: the relationships among dose, effectiveness, and side effects. Chest 2001;119:39S-63S. [Crossref] [PubMed]
- Hirsh J, Weitz JI. New antithrombotic agents. Lancet 1999;353:1431-6. [Crossref] [PubMed]
- Menke J, Lüthje L, Kastrup A, et al. Thromboembolism in atrial fibrillation. Am J Cardiol 2010;105:502-10. [Crossref] [PubMed]
- Ahlehoff O, Gislason G, Lamberts M, et al. Risk of thromboembolism and fatal stroke in patients with psoriasis and nonvalvular atrial fibrillation: a Danish nationwide cohort study. J Intern Med 2015;277:447-55. [Crossref] [PubMed]
- Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation–executive summary. Eur Heart J 2006;27:1979-2030. [Crossref] [PubMed]
- Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016;375:2423-34. [Crossref] [PubMed]
- Yuan L, Bao J, Zhao Z, et al. Transbrachial and femoral artery approach endovascular therapy for flush infrarenal aortic occlusion. Eur J Vasc Endovasc Surg 2014;48:46-52. [Crossref] [PubMed]
- Peeters Weem SM, van Haelst ST, den Ruijter HM, et al. Lack of Evidence for Dual Antiplatelet Therapy after Endovascular Arterial Procedures: A Meta-analysis. Eur J Vasc Endovasc Surg 2016;52:253-62. [Crossref] [PubMed]
- Riegger J, Byrne RA, Joner M, et al. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: a report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. Eur Heart J 2016;37:1538-49. [Crossref] [PubMed]
- Paikin JS, Wright DS, Crowther MA, et al. Triple antithrombotic therapy in patients with atrial fibrillation and coronary artery stents. Circulation 2010;121:2067-70. [Crossref] [PubMed]
- Kinstner CM, Lammer J, Willfort-Ehringer A, et al. Paclitaxel-Eluting Balloon Versus Standard Balloon Angioplasty in In-Stent Restenosis of the Superficial Femoral and Proximal Popliteal Artery: 1-Year Results of the PACUBA Trial. JACC Cardiovasc Interv 2016;9:1386-92. [Crossref] [PubMed]
- Borissoff JI, Spronk HM, ten Cate H. The hemostatic system as a modulator of atherosclerosis. N Engl J Med 2011;364:1746-60. [Crossref] [PubMed]
- Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Hematol 2007;14:55-61. [Crossref] [PubMed]
- Gasic GP, Arenas CP, Gasic TB, et al. Coagulation factors X, Xa, and protein S as potent mitogens of cultured aortic smooth muscle cells. Proc Natl Acad Sci U S A 1992;89:2317-20. [Crossref] [PubMed]
- Ragosta M, Gimple LW, Gertz SD, et al. Specific factor Xa inhibition reduces restenosis after balloon angioplasty of atherosclerotic femoral arteries in rabbits. Circulation 1994;89:1262-71. [Crossref] [PubMed]