The prognostic value of positron emission tomography/computed tomography in pulmonary metastasectomy
Introduction
Despite rapid progress in the field of oncology, metastatic disease remains challenging to treat. The lung is the major target organ of metastases, and pulmonary metastasectomy is a common surgical procedure in thoracic surgery. The data on pulmonary metastasectomy are limited because of the lack of prospective randomized studies and selection bias (1-5). The 5-year overall survival rates have been reported to range from 30% to 50% (3,6,7), and the occurrence of oligometastasis (8,9) suggests the possibility of cure for selected patients with a pulmonary metastasis. Therefore, pulmonary metastasectomy is a common surgical procedure for selected patients with pulmonary metastases.
The main issue of concern for pulmonary metastasectomy is the method of identifying those patients who might obtain survival benefit from pulmonary metastasectomy. Various prognostic factors have been proposed with regard to indications for pulmonary metastasectomy, including the following: disease-free interval (DFI) (6,7), histological features (6), number of metastases (6,7), R0 resection (7), tumor size (9), and metastatic lymph nodes (10-12).
Positron emission tomography/computed tomography (PET/CT) is an imaging technology that uses a radioactive isotope to identify cancer metastases. It is used to estimate the prognosis of patients with various types of malignancies (13). As a method for the preoperative management of patients with primary lung cancer, PET can prevent needless surgery, and PET/CT data from lung cancer patients are used to estimate the prognosis (14,15). Studies on the use of PET/CT for patients with pulmonary metastases are scarce (16-20). We thought that PET/CT could be not only a diagnostic tool (18,19) but also a prognostic tool. Since a high standardized uptake value (SUV) on a PET/CT reflects a higher glucose uptake, it should indicate tumor aggressiveness. To assess PET/CT results for patients with pulmonary metastasis, we conducted a retrospective study of patients with pulmonary metastasis who underwent PET/CT followed by metastasectomy. The aims of this study were to evaluate the value of PET/CT and identify novel prognostic indicators for pulmonary metastasectomy.
Methods
Data collection
Since the patient data remained anonymous, the ethics committee of our institution approved this study (institutional review board no. 29) and waived the need for informed consent. The patient’s personal data have been secured. We consider the study outcomes will not affect the future management of the patients.
This was a retrospective study based on a single-center prospectively collected database of patients undergoing pulmonary metastasectomy from May 2004 to February 2017. Inclusion criteria were as follows: first pulmonary metastasectomy, metastasectomy for curative resection, the patient underwent PET/CT. Our database collected the following: patient characteristics [gender, age, past history, smoking habit, serum carcinoembryonic antigen (CEA) level, preoperative respiratory function], tumor status (size, DFI, tumor laterality and number, SUVmax value), surgical data (date of metastasectomy, surgical procedure, completeness of pulmonary resection), and information on patient outcome (date of death or last confirmed survival). The DFI was calculated from the date of initial treatment for the primary tumor to the date of first detection of pulmonary metastasis.
PET/CT
PET/CT was carried out before surgery within 90 days after the patient had undergone contrast-enhanced chest CT. Since our institution does not have PET/CT on site, starting in 2004, patients were referred to another hospital (Yamagata Saiseikai Hospital or Yamagata University Hospital) to undergo PET/CT. The PET/CT protocol was described previously (21). Patients were instructed to fast for 4 hours before scanning, and a blood glucose level was measured to confirm that it was in the normal range. A standard dose of 18F-fluorodeoxyglucose [FDG (3.75 MBq/kg)] was administered intravenously, and PET and CT images were obtained 60 min later by a Discovery LS instrument (General Electric, Milwaukee, Wisconsin, USA) in Yamagata Saiseikai Hospital. In Yamagata University Hospital, the dose of 18FDG was 3.70 MBq/kg; otherwise the images obtained by the Biograph mCT PET/CT scanner (Simens, München, Germany) at that institution used the same settings and conditions that were used at Yamagata Saiseikai Hospital. Scanning was performed from the base of the skull to the level of the midthigh. To determine the SUVmax after images were obtained, the region of interest, which was established by the radiologists who evaluated the PET scan, was manually placed over the tumor site on each transaxial slice. The SUVmax of each slice was then automatically calculated by software. For patients who had multiple pulmonary metastases, a single lesion with the highest SUVmax was used for analysis.
Patients
Between May 2004 and February 2017, 246 patients underwent metastasectomy for pulmonary metastases. Sixty-eight cases not undergoing PET/CT, 32 undergoing repeated metastasectomy, and 4 undergoing exploratory thoracotomy were excluded. We included 13 patients with incomplete resection who had extrapulmonary metastases or contralateral pulmonary metastasis at the time of PET/CT and pulmonary metastasectomy. A total of 142 patients were eligible for this study. The surgical indication, procedure, and approach for each patient were determined by the consensus of a group of thoracic surgeons. In general, we performed wedge resection for metastatic nodules, and choose segmentectomy for tumors located in the hilum. Lobectomy was selected for multiple nodules located in the same lobe or for tumors >3.0 cm. Lymph node dissection was performed for tumors >3.0 cm. Staged pulmonary metastasectomies were usually performed at 1-month intervals for multiple bilateral nodules. Survival data were updated every 6 months.
Statistical analysis
Receiver operating characteristic (ROC) curve analysis was used to identify the appropriate DFI and SUVmax cut-off values. After each case was classified based on the SUVmax cut-off value, the Chi-square test was used to evaluate the association between each categorical variable and category of SUVmax. The Student t-test was used to compare continuous variables and SUVmax. The median follow-up time was estimated by the reverse Kaplan-Meier method. Overall survival was estimated using the Kaplan-Meier method. Overall survival was measured from the date of pulmonary metastasectomy to the date of death from any cause or censored at the date of the patient’s last hospital visit. Univariable and multivariable Cox proportional hazards regression analysis was used to identify prognostic indicators of overall survival. Data were analyzed using JMP software, version 11.0 (SAS Institute Inc., Cary, NC, USA). A P value <0.05 was considered statistically significant.
Results
There were no perioperative deaths. Major complications were seen in 8 patients (5.6%): prolonged air leakage in 3 (2.1%), bleeding and hemothorax in 2 (1.4%), atrial fibrillation in 1 (0.7%), pulmonary edema in 1 (0.7%), and paralysis of the brachial plexus in 1 (0.7%) patient. Regarding the administration of adjuvant therapy after pulmonary metastasectomy, 35 patients received adjuvant therapy, and 38 patients did not receive it. However, data on adjuvant therapy for 69 patients was not available. The Kaplan-Meier estimate of the median length of follow-up was 42 months. Among these followed patients, 32 patients (22.5%) died (cancer: 26; other causes: 2; unknown cause: 4).
Patient characteristics and association with SUV
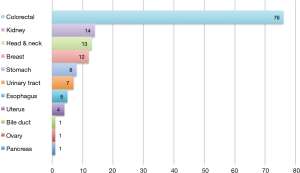
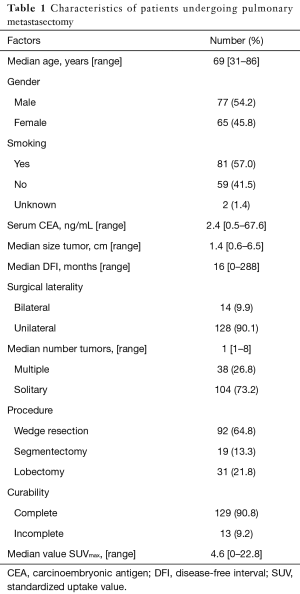
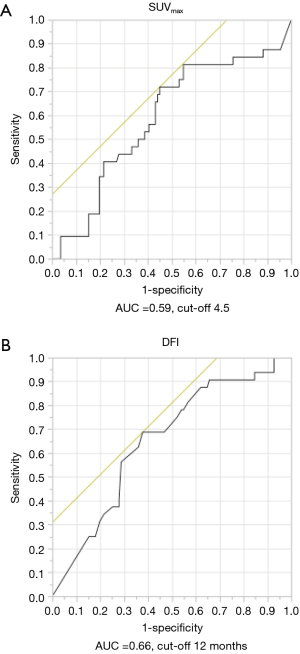
The primary sites were as follows: colorectal in 76 (53.5%), kidney in 14 (9.9%), head and neck in 13 (9.2%), breast in 12 (8.5%), stomach in 8 (5.6%), urinary tract in 7 (4.9%), esophagus in 5 (3.5%), uterus in 4 (2.8%), bile tract in 1 (0.7%), ovary in 1 (0.7%) and pancreas in 1 (0.7%) (Figure 1). Table 1 shows the patients’ demographic data. Almost all cases were unilateral or solitary pulmonary metastases. Wedge resection was the most common procedure. Sublobar resections were performed in 78.1%. The SUVmax cut-off value for survival was 4.5 [area under the curve (AUC) =0.59] by ROC analysis, and the DFI cut-off value was 12 months (AUC =0.66) (Figure 2). Table 2 shows the associations between the patients’ characteristics and SUVmax. The tumor size was larger in cases with SUVmax ≥4.5. The proportion of non-wedge resection cases was higher with SUVmax ≥4.5. Since a large proportion of patients had colorectal primary cancers, the differences between the SUVmax values of patients with colorectal primary cancers and of the values of each group with a non-colorectal primary cancer were evaluated, but there was no difference.
Full table
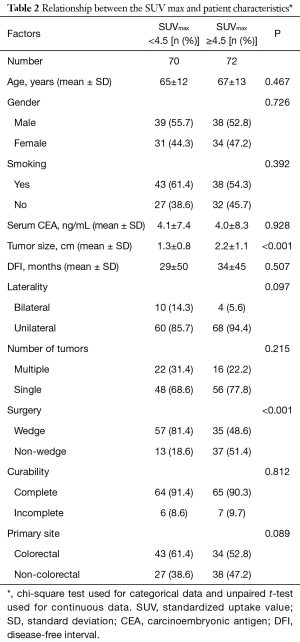
Full table
Survival and recurrence
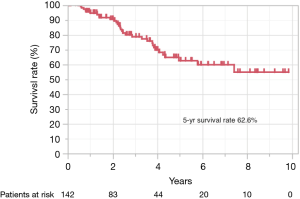
Figure 3 shows the Kaplan-Meier curve of the overall survival of all cases. The overall 5-year survival rate after pulmonary metastasectomy was 62.6%. For univariable and multivariable analysis, patients were classified according to the following categories based on ROC curve analysis: DFI ≤ or >12 months, and SUVmax ≥4.5 or <4.5. At the time of last follow-up, 60 of 142 patients (42.3%) had developed recurrence and 32 (22.5%) had died. Univariable analysis revealed DFI ≤12, incomplete resection, and SUVmax ≥4.5 were significant prognostic indicators for overall survival. By multivariable analysis, the prognostic effects of incomplete resection (P=0.026) and SUVmax ≥4.5 (P=0.035) were maintained after correcting for the other candidate prognostic variables.
Based on SUVmax values, the 5-year survival rates of patients with values SUVmax ≥4.5 and <4.5 were 51.6% and 74.0%, respectively (P=0.022) (Figure 4A). Based on DFIs, the 5-year survival rates of patients with DFI ≤12 and >12 months were 50.6% and 73.9%, respectively (P=0.030) (Figure 4B). Regarding curability, the 5-year survival rates of patients who received complete resection was 66.2%, whereas none of the patients with incomplete resection survived 5 years (Figure 4C).
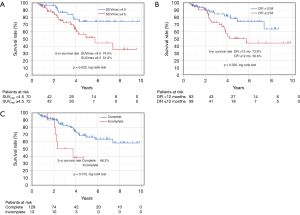
Because of the small study cohort, survival was calculated for the patients with colorectal primaries versus the patients with non-colorectal primaries. Overall survival was similar for the two groups, colorectal vs. non-colorectal patients (P=0.645) (Table 3).
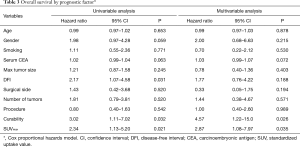
Full table
Discussion
Although the evidence on performing pulmonary metastasectomy for patients with pulmonary metastases is not sufficient, and the selection of patients for pulmonary metastasectomy includes biases (1-5), metastasectomy is a treatment option, and carefully selected patients can obtain a promising outcome compared to primary lung cancer, because the heterogeneity of the outcomes after pulmonary metastasectomy is associated with the types of primaries. The optimization of patient selection is mandatory for achieving the best outcome.
Various types of prognostic indicators have been reported. The International Registry of Lung Metastases previously studied 5,206 patients and found that DFI >3 years and a solitary pulmonary metastasis were favorable prognostic indicators (6). In this study, we identified curability, DFI, and SUVmax. Curability is recognized as an essential prognostic factor. While DFI is also widely accepted, Robert et al. reported that DFI was not prognostic (9). In fact, patients with a slowly growing tumor tend to achieve a long DFI, but DFI depends on the duration of follow-up period and the modalities used for postoperative monitoring. In addition, both CT and PET/CT can identify very small metastatic deposits. However, how do we diagnose small lung nodules as a pulmonary metastasis? Based on this reasoning, we thought that DFI might be controversial in regard to its prognostic value.
Prognostic indicators should be associated with the biological characteristics of the tumor. Tumor doubling time is a prognostic factor related to tumor biology. Chojniak et al. explored the doubling time of pulmonary metastases. They reported that 30% of cases had metastases that did not change in size, and the tumor doubling time varied remarkably among the patients (22). Tumor doubling time requires radiological follow-up after a metastasis is identified, and the tumor doubling time of cases with multiple pulmonary metastases might be difficult to determine.
The SUV is a semiquantitative PET/CT parameter that is calculated based on the accumulation of FDG adjusted by body weight and dose of injected FDG. The SUV is affected by many factors and lacks both reproducibility and standardization. However, it is a useful value for evaluating tumor biology. Promising PET/CT information has been obtained on extra-thoracic malignancies. A systematic review and meta-analysis found that PET/CT is an accurate diagnostic modality for patients with potentially resectable hepatic metastases from colorectal cancer (23). For breast cancer, PET/CT provides benefits for the initial management, and assessments of treatment response and recurrence (24). The SUVmax is correlated with histological characteristics and prognosis (25). A high SUVmax is significant for recurrence in gastric cancer (26), disease-free survival in endometrial cancer (27), and survival in esophageal cancer (28). The SUVmean was associated with disease-free survival in cancers of the head and neck (29).
Studies have been published on the relationship between pulmonary metastasis and PET/CT (16-20). PET/CT of patients with metastatic pulmonary tumor can provide preoperative information that is important for the decision to perform lung surgery. Pastorino et al. revealed that preoperative PET/CT could reduce the number of unnecessary pulmonary metastasectomies (17). PET/CT has been used as a diagnostic tool for differentiating between malignant and benign pulmonary nodules (18,19). Veronesi et al. reported that the SUVmean was 3.9±2.5 (median 3.6) in 43 pulmonary metastases, and higher in colon cancer than in sarcoma metastases, but the difference was not significant (16). Fortes et al. reported that metastasis size was correlated with PET positivity (19), and Pastorino et al. likewise showed that size is related to PET sensitivity (17). PET/CT had not been sufficiently explored with regard to outcomes after pulmonary metastasectomies. Kaira et al. suggested that the T/M ratio, which is the ratio of the peak SUV of the tumor to the mean SUV of the mediastinum, was related to the outcome of patients with a pulmonary metastasis, but only in univariable analysis (20). The present study could show that SUVmax ≥4.5 is significant prognostic indicator. A pulmonary metastasis with an elevated SUV is thought to manifest aggressive biological characteristics. However, PET positivity has not been found to be associated with the intratumoral density of microvessels (16). Further basic research is needed to clarify these relationships.
In this study, the 5-year survival rates of patients with SUVmax ≥4.5 and <4.5 were 51.6% and 74.0%, respectively, which are higher than reported by previous studies (6,7) of patients undergoing pulmonary metastasectomy. We postulate that because PET/CT can detect other distant metastatic lesions and lead to the study exclusion of more advanced cases, our good results might be reflective of stage migration (5).
This study has several limitations. Firstly, the study patients had various types of cancers, which have different biological behaviors and for which PET/CT has shown different sensitivities (16-19). We did not find significant differences in SUVmax values for the survival of patients with colorectal primaries versus non-colorectal primaries. Since half the cases in our study were pulmonary metastases from colorectal cancer, it is possible that we showed the outcome of patients with pulmonary metastases from colorectal cancer. PET studies show that the modality is not sensitive for sarcoma (16), but our study did not include cases of sarcoma and we could not provide data on sarcoma. In addition, renal cell carcinoma generally shows low FDG uptake. However, in our study, the number of cases with renal cell carcinoma was only 14 (10%); therefore, we could not clarify the prognosis of a low FDG uptake in renal cell carcinoma metastases.
The SUVmax might be a unique prognostic factor regardless of the type of primary malignancy; however, we need to collect more data since the numbers of our cases with different types of primary tumor were too small. Moreover, Morris et al. revealed that SUVs of distant metastases might vary based on their location (30). Notably, although the SUVmax of bone metastases was prognostic for patients with newly diagnosed breast cancer, the SUVmax of pulmonary metastases in these patients was not prognostic (30). Secondly, we performed a single-center study, and we could not confirm the reproducibility of our data. To confirm our results, we are planning a prospective study on the effectiveness of SUVmax for patients undergoing pulmonary metastasectomy. Thirdly, the median follow-up time was 42 months, which is not adequate. More than 5 years of follow-up are needed to confirm the benefits of SUVmax for patients undergoing pulmonary metastasectomy (1,7). Fourthly, we used two types of PET/CT scanners because the study patients were seen at two different institutions. Differences between scanners might have affected SUVmax values. To resolve the problem, SUVmax values would have to be corrected. Finally, pulmonary metastasectomy is performed for carefully selected patients. Chemotherapy and other treatments for metastatic cancer enable patients to obtain improved survival. We also did not include patients without surgery, so we do not know if the same results would be obtained for these patients. Given these limitations, our results should be interpreted cautiously; we need more cases to confirm our results.
Conclusions
In conclusion, we found significant correlation between survival and SUVmax values of patients with pulmonary metastasis who underwent resection. However, this is a single-center study of patients with various types of primary malignancies. A prospective observational multicenter study is needed to confirm our results.
Acknowledgements
We are grateful to Yorihisa Watanabe, Nami Watanabe (Yamagata Saiseikai Hospital) and Kazukuni Kirii (Yamagata University Hospital) for providing the PET-CT data.
Footnote
Conflicts of Interest: Presented at the 31st Annual Meeting of the European Association for Cardiothoracic Surgery, Vienna, Austria, 7–11 October 2017.
Ethical Statement: the ethics committee of our institution approved this study (institutional review board no. 29) and waived the need for informed consent.
References
- Aberg T. Selection mechanisms as major determinants of survival after pulmonary metastasectomy. Ann Thorac Surg 1997;63:611-2. [PubMed]
- Treasure T. Pulmonary metastasectomy: a common practice based on weak evidence. Ann R Coll Surg Engl 2007;89:744-48. [Crossref] [PubMed]
- Hornbech K, Ravn J, Steinbrüchel DA. Current status of pulmonary metastasectomy. Eur J Cardiothorac Surg 2011;39:955-62. [Crossref] [PubMed]
- Treasure T, Milosevic M, Fiorentino F, et al. Pulmonary metastasectomy: what is the practice and where is the evidence for effectiveness? Thorax 2014;69:946-9. [Crossref] [PubMed]
- Åberg T, Treasure T. Analysis of pulmonary metastasis as an indication for operation: an evidence-based approach. Eur J Cardiothorac Surg 2016;50:792-8. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. The International Registry of Lung Metastases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Rena O, Papalia E, Oliaro A, et al. Pulmonary metastases from epithelial tumors: late results of surgical treatment. Eur J Cardiothorac Surg 2006;30:217-22. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Robert JH, Ambrogi V, Mermillod B, et al. Factors influencing ling-term survival after lung metastasectomy. Ann Thorac Surg 1997;63:777-84. [Crossref] [PubMed]
- Ercan S, Nichols FC 3rd, Trastek VF, et al. Prognostic significance of lymph node metastasis found during pulmonary metastasectomy for extrapulmonary carcinoma. Ann Thorac Surg 2004;77:1786-91. [Crossref] [PubMed]
- Pfannschmidt J, Klode J, Muley T, et al. Nodal involvement at the time of pulmonary metastasectomy: experiences in 245 patients. Ann Thorac Surg 2006;81:448-54. [Crossref] [PubMed]
- Shiono S, Matsutani N, Okumura S, et al. The prognostic impact of lymph-node dissection on lobectomy for pulmonary metastasis. Eur J Cardiothorac Surg 2015;48:616-21. [Crossref] [PubMed]
- Delbeke D. Oncological application of FDG PET imaging. J Nucl Med 1999;40:1706-15. [PubMed]
- Lardinois D, Weder W, Roudas M, et al. Etiology of solitary extrapulmonary positron emission tomography and computed tomography findings in patients with lung cancer. J Clin Oncol 2005;23:6846-53. [Crossref] [PubMed]
- Berghmans T, Dusart M, Paesmans M, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol 2008;3:6-12. [Crossref] [PubMed]
- Veronesi G, Landoni C, Pelosi G, et al. Fluoro-deoxi-glucose uptake and angiogenesis are independent biological features in lung metastases. Br J Cancer 2002;86:1391-5. [Crossref] [PubMed]
- Pastorino U, Veronesi G, Landoni C, et al. Fluorodeoxyglucose positron emission tomography improves preoperative staging of resectable lung metastasis. J Thorac Cardiovasc Surg 2003;126:1906-10. [Crossref] [PubMed]
- Reinhardt MJ, Wiethoelter N, Matthies A, et al. PET recognition of pulmonary metastases on PET/CT imaging: impact of attenuation-corrected and non-attenuation-corrected images. Eur J Nucl Med Mol Imaging 2006;33:134-9. [Crossref] [PubMed]
- Fortes DL, Allen MS, Lowe VJ, et al. The sensitivity of 18F-fluorodeoxyglucose positron emission tomography in the evaluation of metastatic pulmonary nodules. Eur J Cardiothorac Surg 2008;34:1223-7. [Crossref] [PubMed]
- Kaira K, Okumura T, Ohde Y, et al. Correlation between 18F-FDG uptake on PET and molecular biology in metastatic pulmonary tumors. J Nucl Med 2011;52:705-11. [Crossref] [PubMed]
- Shiono S, Abiko M, Sato T. Positron emission tomography/computed tomography and lymphovascular invasion predict recurrence in stage I lung cancers. J Thorac Oncol 2011;6:43-7. [Crossref] [PubMed]
- Chojniak R, Younes RN. Pulmonary metastases tumor doubling time: assessment by computed tomography. Am J Clin Oncol 2003;26:374-7. [Crossref] [PubMed]
- Wiering B, Krabbe PFM, Jager GJ, et al. The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. A systematic review and meta-analysis. Cancer 2005;104:2658-70. [Crossref] [PubMed]
- Bourgeois AC, Warren LA, Chang TT, et al. Role of positron emission tomography/computed tomography in breast cancer. Radiol Clin N Am 2013;51:781-98. [Crossref] [PubMed]
- Baba S, Isoda T, Maruoka Y, et al. Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: Comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med 2014;55:736-42. [Crossref] [PubMed]
- Lee JW, Lee SM, Lee MS, et al. Role of 18F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur J Nucl Med Mol Imaging 2012;39:1425-34. [Crossref] [PubMed]
- Ghooshkhanei H, Treglia G, Sabouri G, et al. Risk stratification and prognosis determination using (18)F-FDG PET imaging in endometrial cancer patients: a systematic review and meta-analysis. Gynecol Oncol 2014;132:669-76. [Crossref] [PubMed]
- Kato H, Nakajima M, Sohda M, et al. The clinical application of (18)F-fluorodeoxyglucose positron emission tomogramphy to predict survival in patients with operable esophageal cancer. Cancer 2009;115:3196-203. [Crossref] [PubMed]
- Higgins KA, Hoang JK, Roach MC, et al. Analysis of pretreatment FDG-PET SUV parameters in head-and-neck cancer: tumor SUVmean has superior prognostic value. Int J Radiat Oncol Biol Phys 2012;82:548-53. [Crossref] [PubMed]
- Morris PG, Ulaner GA, Eaton A, et al. Standardized uptake value by positron emission tomography/computed tomography as a prognostic variable in metastatic breast cancer. Cancer 2012;118:5454-62. [Crossref] [PubMed]