Division of the intersegmental plane using electrocautery for segmentectomy in clinical stage I non-small cell lung cancer
Introduction
Recently, segmentectomy for small non-small cell lung cancers has been widely performed and the validity of this procedure including video-assisted thoracoscopic surgery (VATS) segmentectomy has been established (1-4). In a systematic meta-analytical review, Cao et al. (5) found no significant difference in overall survival (OS) or disease-free survival (DFS) in the intentional or radical segmentectomy group compared with lobectomy, but significantly worse outcomes for palliative segmentectomy in the compromised group. Meticulous anatomical segmentectomy thus appears central to obtaining feasible outcomes in segmentectomy for lung cancer.
Division of the intersegmental plane is one of the important practical issues for segmentectomy to obtain feasible outcomes without relapse. Almost all surgeons perform this division using staplers. However, division of the intersegmental plane by electrocautery was initially reported by Okada and Tsubota (6,7). We demonstrate herein the merits and drawbacks of division of the intersegmental plane by electrocautery for segmentectomy in clinical stage I non-small cell lung cancer.
Methods
Patients
This study was approved by the institutional review board at the Graduate School of Medicine, Gifu University (approval No. 29-100), and written informed consent was obtained from all patients with the documents of our hospital. Of 686 patients who underwent lobectomy or segmentectomy from 2004 to 2016 (434 men, 252 women; mean age, 69.1±9.6 years) with clinical stage I primary lung cancer, lobectomy was performed in 561 patients, while segmentectomy was performed in 125 patients. Of those 125 patients, 62 (49.6%) underwent intensively radical segmentectomy for pure grand glass nodule (GGN), >50% GGN and <2 cm in diameter, or solid tumors <10 mm in diameter, or as part of a Japanese clinical study (Japan Clinical Oncology Group/West Japan Oncology Group (JCOG0802/WJOG4607L)) (8), and the remaining 63 (50.4%) underwent palliative segmentectomy. Table 1 provides the locations of the affected segments.
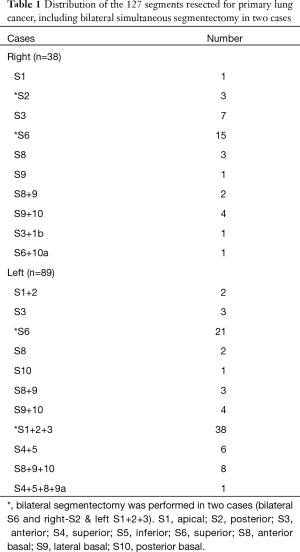
Full table
With our segmentectomy, the intersegmental plane was identified using our original method (9). Briefly, in our surgical procedure, the segmental pulmonary artery and vein are divided. The segment is then inflated with pure oxygen for 5 min. The segmental bronchus is closed by the stapler immediately after oxygen inflation. After a couple of minutes, the intersegmental plane becomes easily detected as an inflation and deflation line. We use electrocauterization or a stapler along this plane for segmental resection. We compared cautery cases (n=50) with stapler cases (n=75). The cautery group included 29 cases (58.0%) with partial use of a staple at the end of division. Delayed air leakage was defined as air leakage that required tube drainage after discharge.
Statistical analysis
We analyzed our data using SPSS software (version 24; SPSS, Chicago, IL, USA). The χ2 test was used to compare the frequencies of categorical variables. Fisher’s exact test was used. OS was defined as the interval from the date of surgery to death from any cause or last follow-up. DFS was defined as the interval from the date of surgery to the first event (relapse or death from any cause) or last follow-up. The Kaplan-Meier method was used to assess OS and DFS, and results were compared using log-rank testing. The level of significance was set at P<0.05.
Results
The proportion of female patients was significantly higher among cautery cases than among stapler cases (P=0.012). Tumor size was significantly smaller in cautery cases (15±6 mm) than in stapler cases (19±8 mm; P=0.003). Tumor standardized uptake value (SUV) from 2-deoxy-2 [18F] fluorodeoxyglucose positron emission tomography (FDG-PET) was significantly lower in cautery cases (1.9±3.4) than in stapler cases (4.2±5.8; P=0.008). Consolidation/tumor (C/T) ratio at the thin-sliced CT was significantly lower in cautery cases (51%±39%) than stapler in cases (70%±36%; P=0.007) (Table 2). Operative time was significantly longer in cautery cases (281±72 min) than stapler in cases (235±86 min; P=0.003). Radical (intensive) cases were significantly more frequent among cautery cases (64.0%) than among stapler cases (40.0%; P=0.009). Pathological stage IA cases were significantly more frequent in cautery cases (94.0%) than in stapler cases (72.0%; P=0.011). Delayed air leakage was significantly more common among cautery cases (7 cases, 14.0%) than among stapler cases (3 cases, 4.0%; P =0.048) (Table 3).
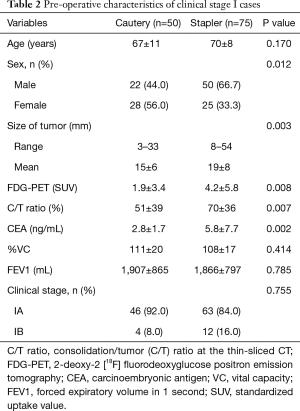
Full table
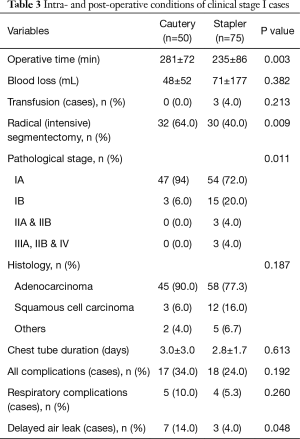
Full table
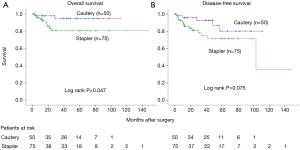
Five-year OS for clinical stage I was 94.7% in cautery cases and 80.5% in stapler cases (log-rank P=0.047). Five-year DFS was 80.0% and 71.3%, respectively (log-rank P=0.075) (Figure 1).
Discussion
In the present study, the 5-year OS was significantly better in cautery cases than in stapler cases (Figure 1). We speculated that this difference was attributable to selection bias according to tumor size, SUV of FDG-PET, carcinoembryonic antigen (CEA), and C/T ratio.
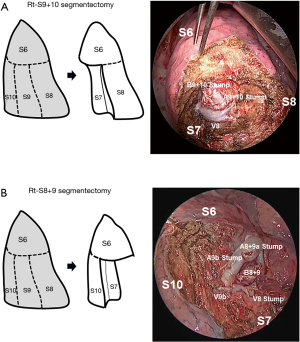
Iwata et al. (9) classified segmentectomy into two types: a simple type, involving resection of only one intersegmental plane, such as superior (S6) or lingual (S4+5) segmentectomy; and a complicated type, involving resection of at least two intersegmental planes, such as anterior (S3) segmentectomy, basal lateral and posterior (S9+10) segmentectomy or basal anterior and lateral (S8+9) segmentectomy. In simple-type segmentectomy, the stapler is quite useful to divide the intersegmental plane quickly and easily. However, residual segments are reshaped by the stapler suture. In complicated-type segmentectomy, such as anterior segmentectomy, stapler division may also be useful, but reshaping of the residual segments occurs. Conversely, division by cautery for complicated-type segmentectomy can achieve the original shape, but air leakage from the intersegmental division surface is an issue that requires attention. In cases such as right basal lateral and posterior (S9+10) segmentectomy (Figure 2A) or basal anterior and lateral (S8+9) segmentectomy (Figure 2B), we have to consider that the right lower lobe contains the medial basal segment (S7). If the intersegmental plane is divided by stapler, the medial basal segment (S7) will probably be damaged by the stapler. We therefore consider that cautery should be used to divide the intersegmental plane in these cases.
Asakura et al. (10) demonstrated, using a porcine model, that the stapler reduced preserved lung expansion compared with cautery or combined methods in segmentectomy. Conversely, Tao et al. (11) compared the influence of methods for segmentectomy on preserved lung volume and pulmonary function between cautery and stapler. They concluded that stapling does not lead to less preserved volume or function than cautery in the division of the intersegmental planes. We think that specific merits and drawbacks exist to division of the intersegmental plane using cautery. Merits include meticulous division of the intersegmental plane and good preservation of the shape of residual segments, especially in complicated-type segmentectomy. Conversely, one key drawback is prolonged air leakage. Regarding air leakage, we compared stapler with cautery division. Operative time was significantly longer in cautery cases than in stapler cases. No difference in duration of chest tube placement was seen between cautery and stapler groups. However, delayed air leakage cases were significantly more frequent in cautery cases than in stapler cases (Table 3). Management of the bare area after division of the intersegmental plane by cautery is thus an important issue.
Regarding management of the intersegmental plane by cautery, two methods are available. One is the pleural closure method of the intersegmental plane with continuous suturing of the pleural edge, such as with a stapler. The other is the mesh-cover method of the intersegmental plane with coverage by polyglycolic acid mesh and fibrin glue (12).
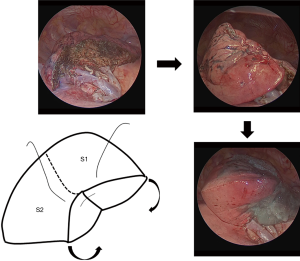
Saito et al. (13) compared postoperative pulmonary function and complications between mesh cover and pleural suture groups. They demonstrated that use of a mesh cover or pleural suture was the only factor independently related to pulmonary complications such as prolonged or delayed air leak after pulmonary segmentectomy. They concluded that pleural suture of the involved intersegmental plane during pulmonary segmentectomy appeared to be an acceptable method. Recently, we have attempted to perform closure of residual segments to prevent prolonged or delayed air leakage (Figure 3). This method seems to obtain good control of postoperative air leakage (data not shown).
We think division with cautery is useful to achieve meticulous, accurate segmentectomy in both complicated-type and sub-segmental resections.
Conclusions
The merits of cautery division include the ability to achieve meticulous division of the intersegmental plane and good preservation of the shape of residual segments. Conversely, the drawbacks include prolonged air leakage. Pleural suture or closure of residual segments may be useful to prevent delayed air leakage.
Acknowledgements
The authors would like to thank Ms Yoshimi Hayashi and Ms Tomoko Kamiya for their secretarial support. This study was supported by grants and endowments from the Department of General and Cardiothoracic Surgery, Graduate School of Medicine, Gifu University.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board at the Graduate School of Medicine, Gifu University (approval No. 29-100), and written informed consent was obtained from all patients with the documents of our hospital.
References
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Okada M. Radical sublobar resection for lung cancer. Gen Thorac Cardiovasc Surg 2008;56:151-7. [Crossref] [PubMed]
- Iwata H. Therapeutic strategy for small-sized lung cancer. Gen Thorac Cardiovasc Surg 2016;64:450-6. [Crossref] [PubMed]
- Gossot D, Lutz J, Grigoroiu M, et al. Thoracoscopic anatomic segmentectomies for lung cancer: technical aspects. J Vis Surg 2016;2:171. [Crossref] [PubMed]
- Cao C, Chandrakumar D, Gupta S, et al. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Tsubota N. An improved method for distinguishing the intersegmental plane of the lung. Surg Today 2000;30:963-4. [Crossref] [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Iwata H, Shirahashi K, Mizuno Y, et al. Surgical technique of lung segmental resection with two intersegmental planes. Interact Cardiovasc Thorac Surg 2013;16:423-5. [Crossref] [PubMed]
- Asakura K, Izumi Y, Kohno M, et al. Effect of cutting technique at the intersegmental plane during segmentectomy on expansion of the preserved segment: comparison between staplers and scissors in ex vivo pig lung. Eur J Cardiothorac Surg 2011;40:e34-8. [Crossref] [PubMed]
- Tao H, Tanaka T, Hayashi T, et al. Influence of stapling the intersegmental planes on lung volume and function after segmentectomy. Interact Cardiovasc Thorac Surg 2016;23:548-52. [Crossref] [PubMed]
- Yoshimoto K, Nomori H, Mori T, et al. Comparison of postoperative pulmonary function and air leakage between pleural closure vs. mesh-cover for intersegmental plane in segmentectomy. J Cardiothorac Surg 2011;6:61. [Crossref] [PubMed]
- Saito H, Konno H, Atari M, et al. Management of Intersegmental Plane on Pulmonary Segmentectomy Concerning Postoperative Complications. Ann Thorac Surg 2017;103:1773-80. [Crossref] [PubMed]


