Cardiac fibrosis in regenerative medicine: destroy to rebuild
Introduction
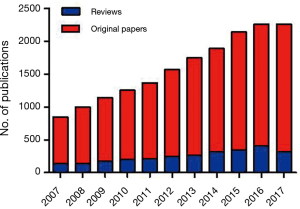
During the last 10 years, cardiac fibrosis has assumed a central role in the cardiovascular field and could be defined as one of the rising topic in the cardiac research. The quantification of scientific papers (original manuscript and reviews) focused on ‘cardiac fibrosis’ present on PubMed database lends credence and veracity to the statement above. The bar graph of Figure 1 indicates the number of publication centred on fibrosis in the heart, which is interestingly almost tripled in the last decade.
Myocardial infarction (MI), together with other detrimental stimuli (i.e., pressure overload), is the starting point of all the adverse structural alterations of extracellular matrix (ECM) occurring in the left ventricular (LV) remodelling (1,2). Since LV pathological changes are due to the post-MI reparative processes (i.e., inflammation, cardiac fibrosis), the study of these events by a cellular and molecular point of view is fundamental for its impact on clinical practice. In fact, it is extremely important to counteract and limit the infarcted area expansion and, to do this, physician routinely perform coronary artery reperfusion (3). Nonetheless a specific therapeutic approach blocking inflammation and the subsequent fibrotic reparative process is still missing (4,5).
The crescent knowledge on the dynamic features of myocardial ECM has unveiled new roads for limiting myocardial fibrosis in terms of type I collagen accumulation and its severe clinical consequences, such as heart failure (HF) (6-9). Interestingly, beyond the structural proteins, ECM contains also non-structural compounds with regulatory roles, such as proteases, which foster changing in collagen structure and content in a rapid temporal fashion (10). Moreover, recent studies have suggested a plethora of potential therapeutic targets that may influence cardiac wound healing and repair (11).
This review is aimed to consider cellular and molecular mediators involved in post-MI repair, pointing out the attention on two protein families that could potentially play a key role in the amelioration of adverse remodelling and cardiac fibrosis development.
Cardiac ventricular fibrosis
Although the process of cardiac fibrosis could be considered an adaptive and protective mechanism, over the time it progresses in an uncontrolled way, driving irreversible remodelling and determining significant impairment in heart function (6).
The ventricular remodelling is a dynamic and complex process resulting from activation of cellular events and molecular pathways which involve several cardiac cell types such as cardiomyocytes, fibroblasts, vascular and immune cells. In pathological conditions, clinical ventricular remodelling may depict three major patterns: (I) a concentric remodelling, when pressure overload determines cardiomyocyte thickening and ECM protein deposition; (II) an eccentric remodelling, resulting from a volume overload that produces cardiomyocyte lengthening; and (III) a post-MI remodelling, which is determined by a combined pressure and volume overload on the non-infarcted area (12). In this review we point out on post-MI remodelling, which occurs after MI damage subsequent to cardiomyocyte necrosis and leads to a wound healing process named reparative fibrosis (13,14). From a molecular point of view, in this type of fibrosis the new deposed ECM, particularly reach in collagen fibres, takes the place of necrotic cells.
A huge number of studies, well reviewed by Prabhu et al. in 2016, have shown the important role played by several molecular and cellular inflammatory mediators in the establishment of the fibrotic process (4). In depth, cardiac repair after MI results from a series of events which begin with an initial phase of sterile inflammation and immune cell infiltration, also called inflammatory phase. This fundamental step leads to digest and clear damaged cells and ECM. A large number of well-known danger-associated molecular patterns is involved in this specific step. Among them, HMGB1, S100 proteins, the extra domain A of fibronectin (ED-A FN), several cytokines and chemokines, such as interleukin (IL)-1α, IL-6, TNF-α are noteworthy (15-18). After that, a reparative phase occurs determining the resolution of the inflammation state, the proliferation of fibroblasts and their differentiation into myofibroblasts (MFB), scar formation and neovascularization (19,20). Although inflammation plays a fundamental role in the progression of post-MI ventricular remodelling, to date is not available a selective therapeutic tool able to effectively turn it off.
Despite the initial trigger leading to fibrous tissue depends on different types of stimulus, there are several molecules leading to increased production of pro-fibrotic mediators, such as the anti-inflammatory IL-10 and the transforming growth factor-β (TGF-β), which acts locally as “master switch” from inflammation to reparative process (12,21). In depth, TGF-β is a pleiotropic cytokine, critically regulating a wide variety of cell functions like growth, proliferation, differentiation, but also ECM deposition. Three structurally similar isoforms of TGF-β (TGF-β1, 2 and 3), encoded by three distinct genes, have been identified in mammalian species (22). TGF-β1 is the prevalent isoform and it has been found almost ubiquitously, whereas the other isoforms are expressed in a more limited spectrum of cells and tissues (21). TGF-β is produced by many cell types and is secreted as a latent small complex (LSC) composed by C-terminal mature TGF-β and its N-terminal pro-domain, the latency-associated peptide (LAP) (23). This complex is further linked to the latent TGF-β binding proteins (LTBP), an ECM fibrillin-like protein family, to form the large latency complex (LLC). Once secreted, LLC is covalently cross-linked to the ECM proteins by the activity of extracellular tissue transglutaminase (24-26). In this conformation, TGF-β is unable to associate with its receptor, so its activation is primarily regulated by its release from the LLC.
Over the past several years, it has been demonstrated that stimuli which can induce protein denaturation (e.g., acid or alkaline pH in extracellular milieu, brief increases in temperature and exposure to oxidants) or proteolysis by the activity of proteases, thrombospondin-1, matrix-metalloproteinase (MMP)-2, and MMP-9 determine the release, and so the activation of TGF-β (27-29). The released TGF-β is able to bind the constitutively active TGF-β type IIreceptor (TβRII). Then, the ligand-receptor complex recruits the type I receptor of TGF-β (TβRI), also known as ALK5, which is expressed by many different cell types. In endothelial cells there is a second TβRI, named ALK1. The activation of both TβRI types, due to their trans-phosphorylation, propagates downstream intracellular signals through the SMAD proteins. While SMAD2 and SMAD3 are activated by ALK5 phosphorylation, SMAD1, SMAD5 and SMAD8 are all activated by ALK1 (30).
To date, it is well-known the key role of TGF–β1 in mediating cardiac hypertrophy (31) by stimulating (I) cardiomyocytes hypertrophy; (II) fibroblast activation and proliferation; and (III) ECM protein synthesis (i.e., collagen) in cardiac tissue (12,32). Notably, the activation of a small fraction of latent TGF-β1 is sufficient to generate maximal cellular response (27). In vitro and in vivo studies with models lacking TGF-β1 strongly contributed to highlight its involvement in several cell functions and, in the meantime, unveiled its pleiotropic role and the great complexity of its management. In this context, gene therapy experiments conducted on a MI model by local transfection of TβRII extracellular domain suggested that early inhibition of TGF-β1 may exacerbate cardiac dysfunctions, while late neutralization of TGF-β signalling may protect from interstitial fibrosis and hypertrophic remodelling (33). Moreover, it has been observed that inhibition of TGF-β after MI resulted in an early mortality caused by cardiac rupture, whereas cardiomyocyte-specific suppression of both TβRI and TβRII stimulated anti-inflammatory and cytoprotective responses (34). Thus, the detrimental effects of early TGF-β inhibition after MI may not lead to a direct action on cardiomyocyte survival, but may determine a loss of anti-inflammatory function on all cardiac cell types (e.g., inflammatory cells, endothelial cells, fibroblasts).
One of the key function of TGF-β1 is the well-known phenotype switch of fibroblast into MFB (35). Morphologically, MFB are characterized by the presence of a contractile apparatus composed by bundles of α-SMA microfilaments and contractile proteins. This apparatus provides a mechano-transduction system able to generate forces by stress fibres, that can be transmitted and transduced by the surrounding ECM into intracellular signals (36-40). To further produce the tension necessary to activate this mechano-transduction, ECM production by MFB is enhanced in the process of remodelling. The most prominent MFB-derived ECM products are type I, III, IV, V, and VI collagen (41). However, the most reliable marker of MFB-derived ECM is the ED-A FN (42) which is also expressed in low amounts by cultured fibroblasts (36,43) and by vascular smooth muscle cells, both in vivo and in vitro (44). Recently, type VI collagen attracted attention as it is up-regulated during myocardial interstitial fibrosis (45) as well as during the fibrotic process in other tissues. It is important to point out that MFB are not present in healthy myocardium, but they are detectable in this region following cardiac injury (46). The origin of MFB in the infarcted area may be ascribed to resident fibroblasts (47) and/or circulating bone marrow progenitors (48), but this remains a debated issue. Precisely, interstitial fibroblasts that survive to ischemic insult and/or cells recruited from neighbouring viable areas may undergo MFB differentiation in response to increased levels of bioactive TGF-β and the subsequent changes in ECM composition. Additional sources of MFB in the healing infarcted area may be represented by endothelial-to-mesenchymal transition of endothelial cells (49), epicardial epithelial cells (50), as well as pericytes (51). Moreover, marked induction of chemokines in response to extensive cardiomyocyte necrosis may result in recruitment and activation of additional subsets of reparative fibroblasts that play an important role in scar formation (4).
Arising targets to limit fibrosis
The amelioration of cell engraftment in cell therapy after MI, to date, is one of the fundamental issue still open in the field of cardiac regenerative medicine. It is noteworthy to mention this matter, since fibrosis plays one of the major guilty role in the incorrect engraftment and subsequent scanty survival of injected cells to treat the impaired cardiac tissue (52,53). In fact, the pathological remodelling of the heart undergoing chronic fibrosis is a significant cause of mortality in cardiovascular disease and there are still no available therapies to reverse or limit its effects (6). Thus, researchers are evaluating novel targets towards the intricate pattern of pathological fibrosis to discover potential therapies in the context of cardiac regeneration. Among the several proteins involved as key participants in the cardiac fibrotic pathways, recently both the integrins and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) proteins have been put in the spotlight. These protein families are strongly related to the TGF-β signalling and involved in the ECM remodelling process which is not only linked to post-MI context, but also to other pathological scenarios (54-57). In particular, the integrins are transmembrane glycoproteins connecting cells with ECM proteins and modulating tissue homeostasis and architecture. The ADAMTS superfamily comprises extracellular enzymes involved in different pathways including maturation of pro-collagen and pathophysiological tissue remodelling (55).
In this review we particularly focus on those proteins belonging to the integrin and ADAMTS families that are expressed in the heart and have the potentiality to counteract the cardiac pathological fibrotic process.
The integrins
The integrins literally constitute the bridge between cells and ECM proteins maintaining a sophisticated structure able to anchor cells and sustain tissue functions in physiological conditions. Nonetheless, integrins are strongly involved in the pathological context of several tissue impairments (58). Integrins are a large family of glycoproteins and act both as adhesive receptors and intracellular signalling events mediators. The integrins are heterodimeric receptors composed by a dimer of an unrelated α and β subunits (59). Currently, in mammalian cells there are 18 α and 8 β known subunits which are able to form at least 24 distinct combination of integrin heterodimers (60). Each subunit contains a large extracellular domain (700–1,100 amino acids), a transmembrane α-helix domain and a short cytoplasmatic tails ranging from 20 to 60 amino acids (61). The integrin binding to ECM glycoproteins, including collagens, fibronectins, laminins, and cellular receptors (i.e., VCAM-1 and ICAMs), is strongly dependent to the presence of divalent cations, typically Ca2+ and Mg2+ (60,62). Concerning molecular functions, integrins participate to a wide range of biological events, including organogenesis, cell-cell and cell-ECM attachment and transduction of signals involved in cell proliferation, differentiation, migration and death. In the cardiovascular system, integrins are expressed in cells of vasculature, blood as well as neurons, cardiac myocytes, and non-muscle cardiac cells. Few studies have shown that integrins are involved in heart formation (63) and function, but also in the development of cardiac diseases (64).
Interestingly, the transcriptional control exerted by TGF-β can strongly affect integrin-mediated processes basically through its regulatory activity on the expression of integrin ligands (i.e., tenascin, vitronectin, fibronectin, members of the laminin and collagen families) and on the stimulation of some integrin-associated protein expression. In 2004, Keski-Oja et al. proposed a non-proteolytic mechanism of TGF-β activation, named “traction model” since it occurs through cell traction forces exerted by the actin cytoskeleton. These forces are translated by integrin into a conformational change of the LLC complex leading to the exposure, and the consequent activation, of TGF-β (65-68). Of note, the LAP of TGF-β1 and TGF-β3, but not TGF-β2, contain the arginine-glycine-aspartate (RGD) motif which can be bound by the αv-containing integrins, αIIbβ3, α5β1 and α8β1 (56). Interestingly, non-proteolytic activation of latent TGF-β has been demonstrated in vitro for αvβ3, αvβ5 and αvβ6, as well as for β1-containing integrins with a still unidentified α-subunit (68). However, the physiological relevance of the TGF-β activation by β1-containing integrins still remains controversial.
Among this large protein family, αv integrins form a subgroup of five members (αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8) all able to recognize a group of overlapping ligands which generally contain the canonical RGD motif (69). These integrins are widely expressed on multiple cell types and, during development, the different αv-associated β subunits show distinct expression patterns (70-72) that, together with the wide range of potential ligands, imply several functions for different receptors. Significant expression of αv integrins has been noted in particular cell types, such as neural crest (73), glial, muscles (70,74,75), and epithelial cells (76,77) as well as osteoclasts (78), blood vessels during development (79-83), and cardiac fibroblasts (84).
Inactivation of αv integrins by αv−/− mice models yielded the death during embryonic development or soon after birth as a result of intracranial and/or gastrointestinal hemorrhage (85). Although the limited survival of mice lacking all αv integrins, the availability of live mice lacking a single αv integrin has made possible a series of studies identifying a number of previously unexpected in vivo roles for these proteins. On the basis of the studies involving knockout mice and/or the use of specific integrin antagonists, are emerging novel insights of this integrin subfamily, which play important and specific roles in (I) determining growth and permeability of blood vessels; (II) regulating tissue inflammation and fibrosis; and (III) developing several common lung diseases (86). Importantly, the αv integrin-mediated activation of TGF-β has been highlighted in vivo by mutation of the RGD site of LAP leading to defects observed in TGF-β1-null mice (87). In addition, genetic ablation of the β6-subunit, or conditional deletion of αv or β8 from dendritic cells, causes exaggerated inflammation as a result of impaired TGF-β signalling (88,89). The phenotype of mice lacking both the αvβ6 and αvβ8 integrins recapitulates the abnormalities observed in TGF-β1 and TGF-β3, but not in TGF-β2, knockout mice, indicating that the integrins αvβ6 and αvβ8 can account for the full activation of TGF-β1 and TGF-β3 in vivo.
The first clue that the integrin-TGF-β interplay is central in fibrosis became from observation on mice lacking the β6-subunit, which are partially or completely protected pulmonary fibrosis induced by radiation or bleomycin (90,91). In addition, low doses of antibodies against αvβ6 prevent radiation- or bleomycin-induced pulmonary fibrosis in mice, without causing inflammation (92,93). Furthermore, it has been shown that constitutive expression of αvβ6 in the basal layer of the epidermis leads to elevated TGF-β1 activation and the development of spontaneous chronic ulcers with severe fibrosis (94). In wild-type mice, fibrosis can be equally inhibited by treatment with antagonists of TGF-β signalling or by using a blocking antibody against αvβ6 (95,96). The αvβ6 integrin is not normally expressed in healthy epithelia, but its expression is induced in several human fibrotic disorders involving kidney, liver and lung in terms of sclerosis and idiopathic pulmonary fibrosis. Specifically, the inhibition of αvβ6-induced TGF-β activation at sites of injury is a promising therapeutic tool to prevent TGF-β-mediated fibrosis.
Mice lacking β3 and/or β5 integrins do not develop abnormalities similar to those caused by TGF-β signalling deficiency (97-99). Nevertheless, αvβ3- and/or αvβ5-mediated TGF-β activation has been reported as an important clue in pathological conditions. In fact, increased expression levels of both these integrins have been observed in dermis of patients with scleroderma, a chronic disease involving cutaneous manifestations of fibrosis. In this pathological context, these integrins elicit in vitro autocrine TGF-β signalling in patient-derived fibroblasts (100-103). The TGF-β activation by αvβ5 is important also in pulmonary fibrosis, whereas the contribution of αvβ3 in this human pathology has not been yet established. In human fibrotic lungs, epithelial cells expressing αvβ5 and the protease activated receptor 1 co-localize with MFB, and TGF-β-mediated pulmonary fibrosis is reduced by the blockade of αvβ5 in a mouse model (104).
Astoundingly, Henderson et al. reported that αv-containing integrins on MFB are components of a core cellular and molecular pathway contributing to pathological fibrosis in multiple solid organs and suggested that the targeting of this pathway could lead clinical utility in the treatment of patients with a broad range of fibrotic diseases (105).
As previously mentioned, MFB are not present in healthy adult myocardium and appear after cardiac injury (106). It has been also reported that MFB result from the differentiation of resident cardiac fibroblasts (107) or from the trans-differentiation of endothelial cells (49,108). The endothelial-to-mesenchymal transition can be induced by TGF-β in a SMAD-dependent fashion during cardiac fibrosis, while BMP-7 is able to block this process acting as an anti-fibrotic factor (49).
Data from preclinical models suggest that integrin-mediated TGF-β activation is involved in several pathological conditions, such as scleroderma, lung, kidney and liver fibrosis (87). In particular, in vitro studies demonstrated that both integrin αvβ6 and αvβ8-mediated TGF-β activation play a key role in lung fibrosis. Indeed, the first one promotes squamous metaplasia of airway epithelial cells (109) while the second inhibits airway epithelial proliferation and migration (110). These results were confirmed by using β6 integrin subunit-null mouse model and airway fibroblast isolated from patients with chronic obstructive pulmonary disease (92). Moreover, it has been shown that the expression of αvβ6 is increased in the migrating epidermal cells adjacent to wound (111), suggesting that this integrin is also involved in skin fibrosis, as confirm by in vivo studies using αvβ6-deficient aged mice (94,112). In addition, several evidences reported that also αvβ3 and αvβ5 integrins, which are able to activate TGF-β in primary culture isolated from scleroderma fibroblasts, are increased in the dermal fibroblasts of biopsy sample from scleroderma patients (100,101,103). Furthermore, Hahm et al. demonstrated that αvβ6 enhances renal fibrosis by using Col4a3−/− mice deficient in αvβ6 integrin or, alternatively, Col4a3−/− mice treated with anti-β6 integrin-blocking antibodies (or a soluble TβRII) (95).
Taken this large amount of data together, it is clear that all these features render nowadays the integrins one of the most interesting therapeutic target on which investigate in the field of specific fibrotic disorders (113). Proofs of this sentence may be appreciated by the large literature, ultimately and notably reviewed in this therapeutic context by Hatley and colleagues in 2017 (114). Although the integrin family was initially identified to have a key role in mediating cell adhesion, it is becoming even more clear that a subset of integrin plays the role of the culprit along with TGF-β in the fibrotic process.
The ADAMTS proteins
The ADAMTS proteins are members of a superfamily that includes 19 metalloproteases and a subfamily composed by 7 ADAMTS lacking their catalytic activity, called ADAMTS-like (ADAMTSL) proteins (55). While the ADAMTS are involved in different pathways, including maturation of pro-collagen and tissue remodelling in several pathophysiological conditions (i.e., angiogenesis, arthritis), the ADAMTSL are component of the ECM with functions potentially linked to the regulation of ADAMTS protein activity (57).
In the cardiovascular context, the role of ADAMTS proteins is arousing growing interest since some of them have been recently found in the culprit plaques of patients with MI and others show versican cleaving activity (6,115-117). Specifically, an up-regulation of ADAMTS2, ADAMTS3, and ADAMTS13 have been observed in coronary lesions of patients with MI highlighting their possible participation in cardiovascular disease (116). Interestingly, both the ADAMTS2 and the ADAMTS3 have pro-collagen N-propeptidase activity, while the ADAMTS13 is a von Willebrand factor cleaving protease. ADAMTS2 is expressed in several tissues, in addition to the heart, and it is involved in multiple processes. Among them, ADAMTS2 primary function is to activate the types I, II, III and V pro-collagen by the promotion of their cleavage (118). Mutations in ADAMTS2 determine enzyme malfunction and cause the Ehlers-Danlos syndrome type VIIC, a rare connective tissue disorder determined by the failure of type I pro-collagen cleavage (119). Dong et al. reported in the fibrotic pathological context of hepatic cirrhosis the pro-fibrotic role of ADAMTS2 where resulted highly expressed and positively correlated with TGF-β levels. In turn, TGF-β probably induces ADAMTS2 expression through the SMAD signalling (120). Conversely, Wang et al. recently evidenced a protective role for ADAMTS2 in rat angiotensin II-dependent cardiomyocyte hypertrophy and in a murine model of cardiac hypertrophy induced by pressure overload. Moreover, they also demonstrated in patients with dilated cardiomyopathy that cardiac hypertrophy is hampered by ADAMTS2 expression levels (118). Indeed, under pathological conditions ADAMTS2 is able to protect the heart preventing hypertrophy by the inactivation of the PI3K/AKT signalling pathway, a recognized key mediator of cardiac hypertrophy. For this role in modulating cardiac fibrosis and its easy and effective manipulation as extracellular protein, ADAMTS2 has been proposed as novel pharmacological tool (118).
Among the three proteins found up-regulated in the coronary lesions of patients with MI, ADAMTS3 plays the most complex role due to its involvement in several biological processes not always related to collagen maturation (i.e., blood coagulation, neoangiogenesis, development, male fertility). To our knowledge its role in cardiovascular fibrosis have not been already clarified (121).
Although ADAMTS13 is principally related to the development of thrombotic thrombocytopenic purpura—a rare disease characterized by thrombocytopenia, hemolytic anemia, and thrombi formation in the microvasculature—there is increasing evidence that its malfunctioning has a role in adverse cardiovascular events, including HF (122-127). Interestingly, the peculiar von Willebrand factor cleavage activity of ADAMTS13 has been recently exploit to investigate its effect on chronic myocardial injury in a pressure overload mouse model. Specifically, the recombinant human ADAMTS13 administration determined in vivo a strong improvement in myocardial remodelling and functionality due to the ADAMTS13 action in preventing inflammation, platelet recruitment and microvessel obstruction (128). Of note, the recombinant human ADAMTS13 is currently used in a clinical trial for the treatment of thrombotic thrombocytopenic purpura, so it may be exploitable as a new therapeutic tool against fibrotic cardiac damage (129).
Another interesting function attractive for researchers regards ADAMTS1 and ADAMTS4 which are capable to cleave versican, the primary proteoglycan component of the vasculature. In the cardiovascular context, ADAMTS1 and ADAMTS4 ability in ECM rearrangement results crucially detrimental both for the ventricular remodelling after MI and the regulation of fibrous cap stability in atherosclerotic plaque (117,130). The damaging role of ADAMTS1 is basically due to its cleavage activity of versican that, once cleaved, stimulates vascular smooth muscle cell migrations (131). Besides, ADAMTS1 has many beneficial functions including anti-angiogenic activity, versican turnover during mouse cardiac development, and type I collagen degrading activity (57,132-135). Interestingly, the ability of ADAMTS1 to degrade type I collagen has been recently demonstrated in a mouse model of chronic viral myocarditis (CVMC), a disease that, at chronic stage, is characterized by ECM accumulation in heart tissue contributing to cardiac function loss (115). In this context, ADAMTS1 myocardial protein expression has been found to be inversely correlated with the expression levels of type I collagen, and positively correlated with the carboxyterminal telopeptide of type I collagen, a degradation marker of type I collagen, released during its breakdown (115). Furthermore, in vitro studies provided evidence that, during the progression of CVMC to dilated cardiomyopathy, IL-17 as well as TGF-β are able to up-regulate ADAMTS1 expression, increasing type I collagen degradation (136). The same function of ADAMTS1 has been demonstrated in murine CVMC model after treatment with the angiotensin II converting enzyme inhibitor (ACEi) captopril. In this scenario ADAMTS1 contributes to the anti-fibrotic effect of captopril by accelerating type I collagen degradation (115). Although nowadays the exact underlying mechanism remained to be determined, the link between ACEi and ADAMTS1 opens new perspectives for potential combined therapy (115,137).
Concerning ADAMTS4, it has a fundamental role in degradation versican and aggrecan and thus it is counted among the pro-fibrotic ADAMTS proteins (117,138). This is basically because a link between the ADAMTS4 activity and HF development has been described (138). In depth, in the myocardium of rats subjected to pressure overload by aortic banding was observed an increased ADAMTS4 versicanase activity. Furthermore, the inhibition of ADAMTS4 expression and activity by pentosan polysulfate (PPS) treatment improved cardiac contractile performance (138). On the basis of these discoveries together with the proven positive effect of PPS in reducing the infarct size in reperfusion models, the inhibition of ADAMTS4 is now depicted as a promising novel therapeutic approach in HF. Of note, the modulation of ADAMTS4 activity could be of great importance also in the atherosclerotic context where TGF-β-dependent inhibition of ADAMTS4 secretion by macrophages contributes to plaque stabilization (117).
Another member of the ADAMTS family linked to TGF-β signalling is ADAMTS5 which is principally involved in cartilage aggrecanase activity and joint fibrosis (139,140). In detail, ADAMTS5 determines in vivo the balance of proteoglycan turnover in the derma and, when absent, the TGF-β signalling intensification (140). In murine model of atherosclerosis ADAMTS5 promotes also lipoprotein retention. It has been properly found that ADAMTS5 determines the physiological release of versican and aggrecan fragments, and that its reduction is accompanied by the accumulation of biglycan and versican, the major LDL-binding species (141).
Interestingly, a study using balloon-injured arteries of rats showed that ADAMTS7 determines vascular smooth muscle cell migration and neointima formation probably through the degradation of thrombospondin-5, while another study in mouse model indicated the thrombospondin-1 as principal mediator of neointima formation, determining a retarded re-endothelialization (142,143). Furthermore, it has been also showed that ADAMTS7 accumulates in the smooth muscle cells of coronary and carotid atherosclerotic plaques (144). A new link between ADAMTS7 and the cardiovascular context has been recently drawn by the association between its plasmatic levels and the worsening of LV function in patients with MI. Precisely, in a prospective study conducted by Wu et al. on STEMI, non-STEMI patients and controls it has been found that ADAMTS7 levels were greater in patients with LV ejection fraction ≤35%, independently from the STEMI or non-STEMI diagnosis (145). Despite all these results elect ADAMTS7 as a new target for possible post injury vascular intima hyperplasia treatments, further studies are needed to verify its exact mechanism of action in LV remodelling.
Among the ADAMTS family there are also ‘sister proteins’ involved in the same pathways, but determining opposite effects. This is the case of ADAMTS10 and ADAMTS6, both involved in fibrillin-1 microfibril formation, but with opposite functions. ADAMTS10 is required for focal adhesions, epithelial cell-cell junction formation, and microfibril deposition and it is known to cause, when mutated, the Weill-Marchesani syndrome, while ADAMTS6 has an inhibitory effect on the same pathways (146).
The previously mentioned ADAMTSL proteins are integral components of the ECM and some of them, such as ADAMTSL2, ADAMTSL6 and ADAMTSL4, are interestingly involved in fibrosis. Specifically, ADAMTSL2, when mutated, is the cause of geleophysic dysplasia, an autosomal rare disorder mainly characterized by cardiac valvular thickening and progressive HF leading to premature death. From a molecular point of view, this pathology is characterized by an enhanced ECM mechanical stability (147). In particular, ADAMTSL2 binds both fibrillin-1 and the LTBP-1 determining the increase in the TGF-β activity levels typical of this pathology.
Moving from the cardiovascular to a general context, ADAMTSL6 was found functionally involved in the organization of the ECM in mice due to its capacity to directly bind fibrillin-1 and to promote its matrix assembly. Interestingly, ADAMTSL6 is highly expressed in murine heart tissue and its direct binding to fibrillin-1 has been demonstrated by surface plasmon resonance binding assay. Moreover, ADAMTSL6 overexpression in transgenic mice determined an excessive fibrillin-1 microfibril formation (148).
For another member of the subfamily, ADAMTSL4, has been described a role strictly related to fibrillin-1 assembly and function in patients affected by ectopia lentis (149). In particular, the dislocation of the ocular lens in these subjects is determined by failed maintenance of the lens in the correct position because of the laxity of their suspensory ligaments principally composed by fibrillin-1. Of interest, mutations in several ADAMTS/ADAMTSL proteins affect the regulation of microfibrils in terms of assembly, stability and anchorage, resembling the phenotype observed in some fibrillin-1 related genetic disorders like geleophysic dysplasia, the Weill-Marchesani and Marfan syndrome, further confirming their functional link with microfibril network (6,150,151).
In conclusion, since several studies on ADAMTS/ADAMTSL proteins provided evidence of their involvement in cardiac fibrosis they may serve as promising targets to be boosted (i.e., ADAMTS2, ADAMTS13) or inhibited (i.e., ADAMTS4) on the basis of their main role for preventing cardiac hypertrophy and HF.
Conclusions
To date, the knowledge of all molecular mediators involved in heart injury, repair, and remodelling after MI has unveiled a series of new possible therapeutic targets supporting cell therapy for patients. In fact, because of the fibrosis, the post-MI cardiac milieu is a discomfort zone to receive and embrace protective and potentially reparative cells. Pharmacological studies are now needed to define the exact effect of novel tools on pathological alterations leading to adverse remodelling after MI. Strategies able to modulate the fibrotic process are not only necessary to reduce, or even avoid, cardiac remodelling and subsequent HF, but may be also crucial in the future obtainment of effective cell-based myocardial regeneration.
Acknowledgements
Funding: This work was supported by Ministero della Salute (RC 2017/18 to Centro Cardiologico Monzino—IRCCS).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Prabhu SD. Post-infarction ventricular remodeling: an array of molecular events. J Mol Cell Cardiol 2005;38:547-50. [Crossref] [PubMed]
- Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 2000;101:2981-8. [Crossref] [PubMed]
- Braunwald E. The treatment of acute myocardial infarction: the Past, the Present, and the Future. Eur Heart J Acute Cardiovasc Care 2012;1:9-12. [Crossref] [PubMed]
- Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res 2016;119:91-112. [Crossref] [PubMed]
- Fernandez-Aviles F, Sanz-Ruiz R, Climent AM, et al. Global position paper on cardiovascular regenerative medicine. Eur Heart J 2017;38:2532-46. [Crossref] [PubMed]
- Murtha LA, Schuliga MJ, Mabotuwana NS, et al. The Processes and Mechanisms of Cardiac and Pulmonary Fibrosis. Front Physiol 2017;8:777. [Crossref] [PubMed]
- Querejeta R, Lopez B, Gonzalez A, et al. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation 2004;110:1263-8. [Crossref] [PubMed]
- Satoh M, Nakamura M, Akatsu T, et al. Myocardial osteopontin expression is associated with collagen fibrillogenesis in human dilated cardiomyopathy. Eur J Heart Fail 2005;7:755-62. [Crossref] [PubMed]
- Gambini E, Perrucci GL, Bassetti B, et al. Preferential myofibroblast differentiation of cardiac mesenchymal progenitor cells in the presence of atrial fibrillation. Transl Res 2018;192:54-67. [Crossref] [PubMed]
- Goldsmith EC, Bradshaw AD, Spinale FG. Cellular mechanisms of tissue fibrosis. 2. Contributory pathways leading to myocardial fibrosis: moving beyond collagen expression. Am J Physiol Cell Physiol 2013;304:C393-402. [Crossref] [PubMed]
- Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol 2015;173:370-8. [Crossref] [PubMed]
- Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 2007;74:184-95. [Crossref] [PubMed]
- Shirwany A, Weber KT. Extracellular matrix remodeling in hypertensive heart disease. J Am Coll Cardiol 2006;48:97-8. [Crossref] [PubMed]
- Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol 1989;13:1637-52. [Crossref] [PubMed]
- Schiraldi M, Raucci A, Munoz LM, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 2012;209:551-63. [Crossref] [PubMed]
- Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med 2011;17:1391-401. [Crossref] [PubMed]
- Timmers L, Pasterkamp G, de Hoog VC, et al. The innate immune response in reperfused myocardium. Cardiovasc Res 2012;94:276-83. [Crossref] [PubMed]
- de Haan JJ, Smeets MB, Pasterkamp G, et al. Danger signals in the initiation of the inflammatory response after myocardial infarction. Mediators Inflamm 2013;2013. [Crossref] [PubMed]
- Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res 2012;110:159-73. [Crossref] [PubMed]
- Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 2010;121:2437-45. [Crossref] [PubMed]
- Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol 2011;51:600-6. [Crossref] [PubMed]
- Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci 2004;35:83-92. [Crossref] [PubMed]
- Koli K, Saharinen J, Hyytiainen M, et al. Latency, activation, and binding proteins of TGF-beta. Microsc Res Tech 2001;52:354-62. [Crossref] [PubMed]
- Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 2007;8:970-82. [Crossref] [PubMed]
- Ashcroft GS, Yang X, Glick AB, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol 1999;1:260-6. [Crossref] [PubMed]
- Roncoroni L, Elli L, Bardella MT, et al. Extracellular matrix proteins and displacement of cultured fibroblasts from duodenal biopsies in celiac patients and controls. J Transl Med 2013;11:91. [Crossref] [PubMed]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci 2003;116:217-24. [Crossref] [PubMed]
- Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev 2000;11:59-69. [Crossref] [PubMed]
- Mu D, Cambier S, Fjellbirkeland L, et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol 2002;157:493-507. [Crossref] [PubMed]
- Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol 2010;48:504-11. [Crossref] [PubMed]
- Almendral JL, Shick V, Rosendorff C, et al. Association between transforming growth factor-beta(1) and left ventricular mass and diameter in hypertensive patients. J Am Soc Hypertens 2010;4:135-41. [Crossref] [PubMed]
- Dobaczewski M, Frangogiannis NG. Chemokines and cardiac fibrosis. Front Biosci (Schol Ed) 2009;1:391-405. [Crossref] [PubMed]
- Ikeuchi M, Tsutsui H, Shiomi T, et al. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc Res 2004;64:526-35. [Crossref] [PubMed]
- Rainer PP, Hao S, Vanhoutte D, et al. Cardiomyocyte-specific transforming growth factor beta suppression blocks neutrophil infiltration, augments multiple cytoprotective cascades, and reduces early mortality after myocardial infarction. Circ Res 2014;114:1246-57. [Crossref] [PubMed]
- Desmouliere A, Geinoz A, Gabbiani F, et al. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993;122:103-11. [Crossref] [PubMed]
- Dugina V, Fontao L, Chaponnier C, et al. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J Cell Sci 2001;114:3285-96. [PubMed]
- Gabbiani G, Hirschel BJ, Ryan GB, et al. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med 1972;135:719-34. [Crossref] [PubMed]
- Willems IE, Havenith MG, De Mey JG, et al. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol 1994;145:868-75. [PubMed]
- Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc Res 2000;48:89-100. [Crossref] [PubMed]
- Turner NA, Porter KE. Function and fate of myofibroblasts after myocardial infarction. Fibrogenesis Tissue Repair 2013;6:5. [Crossref] [PubMed]
- Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol 2007;127:526-37. [Crossref] [PubMed]
- Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res 1999;250:273-83. [Crossref] [PubMed]
- Hinz B, Celetta G, Tomasek JJ, et al. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 2001;12:2730-41. [Crossref] [PubMed]
- Glukhova MA, Frid MG, Shekhonin BV, et al. Expression of extra domain A fibronectin sequence in vascular smooth muscle cells is phenotype dependent. J Cell Biol 1989;109:357-66. [Crossref] [PubMed]
- Kitamura M, Shimizu M, Ino H, et al. Collagen remodeling and cardiac dysfunction in patients with hypertrophic cardiomyopathy: the significance of type III and VI collagens. Clin Cardiol 2001;24:325-9. [Crossref] [PubMed]
- Fan D, Takawale A, Lee J, et al. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair 2012;5:15. [Crossref] [PubMed]
- Yano T, Miura T, Ikeda Y, et al. Intracardiac fibroblasts, but not bone marrow derived cells, are the origin of myofibroblasts in myocardial infarct repair. Cardiovasc Pathol 2005;14:241-6. [Crossref] [PubMed]
- Mollmann H, Nef HM, Kostin S, et al. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res 2006;71:661-71. [Crossref] [PubMed]
- Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 2007;13:952-61. [Crossref] [PubMed]
- Ruiz-Villalba A, Simon AM, Pogontke C, et al. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J Am Coll Cardiol 2015;65:2057-66. [Crossref] [PubMed]
- Hinz B, Phan SH, Thannickal VJ, et al. The myofibroblast: one function, multiple origins. Am J Pathol 2007;170:1807-16. [Crossref] [PubMed]
- Dai B, Huang W, Xu M, et al. Reduced collagen deposition in infarcted myocardium facilitates induced pluripotent stem cell engraftment and angiomyogenesis for improvement of left ventricular function. J Am Coll Cardiol 2011;58:2118-27. [Crossref] [PubMed]
- Nigro P, Bassetti B, Cavallotti L, et al. Cell therapy for heart disease after 15 years: Unmet expectations. Pharmacol Res 2018;127:77-91. [Crossref] [PubMed]
- Chen C, Li R, Ross RS, et al. Integrins and integrin-related proteins in cardiac fibrosis. J Mol Cell Cardiol 2016;93:162-74. [Crossref] [PubMed]
- Kelwick R, Desanlis I, Wheeler GN, et al. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol 2015;16:113. [Crossref] [PubMed]
- Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep 2010;11:97-105. [Crossref] [PubMed]
- Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem 2009;284:31493-7. [Crossref] [PubMed]
- Karsdal MA, Manon-Jensen T, Genovese F, et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am J Physiol Gastrointest Liver Physiol 2015;308:G807-30. [Crossref] [PubMed]
- Hynes RO. Integrins: a family of cell surface receptors. Cell 1987;48:549-54. [Crossref] [PubMed]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002;110:673-87. [Crossref] [PubMed]
- Humphries MJ. Integrin structure. Biochem Soc Trans 2000;28:311-39. [Crossref] [PubMed]
- Plow EF, Haas TA, Zhang L, et al. Ligand binding to integrins. J Biol Chem 2000;275:21785-8. [Crossref] [PubMed]
- Baldwin HS, Buck CA. Integrins and other cell adhesion molecules in cardiac development. Trends Cardiovasc Med 1994;4:178-87. [Crossref] [PubMed]
- Ross RS. Molecular and mechanical synergy: cross-talk between integrins and growth factor receptors. Cardiovasc Res 2004;63:381-90. [Crossref] [PubMed]
- Keski-Oja J, Koli K, von Melchner H. TGF-beta activation by traction? Trends Cell Biol 2004;14:657-9. [Crossref] [PubMed]
- Annes JP, Chen Y, Munger JS, et al. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol 2004;165:723-34. [Crossref] [PubMed]
- Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol 2008;87:601-15. [Crossref] [PubMed]
- Wipff PJ, Rifkin DB, Meister JJ, et al. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 2007;179:1311-23. [Crossref] [PubMed]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 1992;69:11-25. [Crossref] [PubMed]
- Hirsch E, Gullberg D, Balzac F, et al. Alpha v integrin subunit is predominantly located in nervous tissue and skeletal muscle during mouse development. Dev Dyn 1994;201:108-20. [Crossref] [PubMed]
- Alfandari D, Whittaker CA, DeSimone DW, et al. Integrin alpha v subunit is expressed on mesodermal cell surfaces during amphibian gastrulation. Dev Biol 1995;170:249-61. [Crossref] [PubMed]
- Yamada KM, Miyamoto S. Integrin transmembrane signaling and cytoskeletal control. Curr Opin Cell Biol 1995;7:681-9. [Crossref] [PubMed]
- Delannet M, Martin F, Bossy B, et al. Specific roles of the alpha V beta 1, alpha V beta 3 and alpha V beta 5 integrins in avian neural crest cell adhesion and migration on vitronectin. Development 1994;120:2687-702. [PubMed]
- McDonald KA, Lakonishok M, Horwitz AF. Alpha v and alpha 3 integrin subunits are associated with myofibrils during myofibrillogenesis. J Cell Sci 1995;108:975-83. [PubMed]
- Martin PT, Sanes JR. Integrins mediate adhesion to agrin and modulate agrin signaling. Development 1997;124:3909-17. [PubMed]
- Breuss JM, Gallo J, DeLisser HM, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 1995;108:2241-51. [PubMed]
- Huang XZ, Wu JF, Cass D, et al. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol 1996;133:921-8. [Crossref] [PubMed]
- Horton MA. Interactions of connective tissue cells with the extracellular matrix. Bone 1995;17:51S-3S. [Crossref] [PubMed]
- Brooks PC, Montgomery AM, Rosenfeld M, et al. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994;79:1157-64. [Crossref] [PubMed]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994;264:569-71. [Crossref] [PubMed]
- Drake CJ, Cheresh DA, Little CD. An antagonist of integrin alpha v beta 3 prevents maturation of blood vessels during embryonic neovascularization. J Cell Sci 1995;108:2655-61. [PubMed]
- Friedlander M, Brooks PC, Shaffer RW, et al. Definition of two angiogenic pathways by distinct alpha v integrins. Science 1995;270:1500-2. [Crossref] [PubMed]
- Friedlander M, Theesfeld CL, Sugita M, et al. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc Natl Acad Sci U S A 1996;93:9764-9. [Crossref] [PubMed]
- Graf K, Neuss M, Stawowy P, et al. Angiotensin II and alpha(v)beta(3) integrin expression in rat neonatal cardiac fibroblasts. Hypertension 2000;35:978-84. [Crossref] [PubMed]
- Bader BL, Rayburn H, Crowley D, et al. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell 1998;95:507-19. [Crossref] [PubMed]
- Sheppard D. Roles of alphav integrins in vascular biology and pulmonary pathology. Curr Opin Cell Biol 2004;16:552-7. [Crossref] [PubMed]
- Yang L, Chan T, Demare J, et al. Healing of burn wounds in transgenic mice overexpressing transforming growth factor-beta 1 in the epidermis. Am J Pathol 2001;159:2147-57. [Crossref] [PubMed]
- Lacy-Hulbert A, Smith AM, Tissire H, et al. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A 2007;104:15823-8. [Crossref] [PubMed]
- Travis MA, Reizis B, Melton AC, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature 2007;449:361-5. [Crossref] [PubMed]
- Munger JS, Huang X, Kawakatsu H, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319-28. [Crossref] [PubMed]
- Ma LJ, Yang H, Gaspert A, et al. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(-/-) mice. Am J Pathol 2003;163:1261-73. [Crossref] [PubMed]
- Puthawala K, Hadjiangelis N, Jacoby SC, et al. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med 2008;177:82-90. [Crossref] [PubMed]
- Horan GS, Wood S, Ona V, et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med 2008;177:56-65. [Crossref] [PubMed]
- Hakkinen L, Koivisto L, Gardner H, et al. Increased expression of beta6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol 2004;164:229-42. [Crossref] [PubMed]
- Hahm K, Lukashev ME, Luo Y, et al. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 2007;170:110-25. [Crossref] [PubMed]
- Wang B, Dolinski BM, Kikuchi N, et al. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology 2007;46:1404-12. [Crossref] [PubMed]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest 1999;103:229-38. [Crossref] [PubMed]
- Huang X, Griffiths M, Wu J, et al. Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol Cell Biol 2000;20:755-9. [Crossref] [PubMed]
- Reynolds LE, Wyder L, Lively JC, et al. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med 2002;8:27-34. [Crossref] [PubMed]
- Asano Y, Ihn H, Yamane K, et al. Increased expression of integrin alphavbeta5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol 2006;168:499-510. [Crossref] [PubMed]
- Asano Y, Ihn H, Jinnin M, et al. Involvement of alphavbeta5 integrin in the establishment of autocrine TGF-beta signaling in dermal fibroblasts derived from localized scleroderma. J Invest Dermatol 2006;126:1761-9. [Crossref] [PubMed]
- Asano Y, Ihn H, Yamane K, et al. Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J Immunol 2005;175:7708-18. [Crossref] [PubMed]
- Asano Y, Ihn H, Yamane K, et al. Involvement of alphavbeta5 integrin-mediated activation of latent transforming growth factor beta1 in autocrine transforming growth factor beta signaling in systemic sclerosis fibroblasts. Arthritis Rheum 2005;52:2897-905. [Crossref] [PubMed]
- Scotton CJ, Krupiczojc MA, Konigshoff M, et al. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Invest 2009;119:2550-63. [PubMed]
- Henderson NC, Arnold TD, Katamura Y, et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med 2013;19:1617-24. [Crossref] [PubMed]
- Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol 2011;57:376-9. [Crossref] [PubMed]
- Lucas JA, Zhang Y, Li P, et al. Inhibition of transforming growth factor-beta signaling induces left ventricular dilation and dysfunction in the pressure-overloaded heart. Am J Physiol Heart Circ Physiol 2010;298:H424-32. [Crossref] [PubMed]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012;18:1028-40. [Crossref] [PubMed]
- Araya J, Cambier S, Markovics JA, et al. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest 2007;117:3551-62. [Crossref] [PubMed]
- Fjellbirkeland L, Cambier S, Broaddus VC, et al. Integrin alphavbeta8-mediated activation of transforming growth factor-beta inhibits human airway epithelial proliferation in intact bronchial tissue. Am J Pathol 2003;163:533-42. [Crossref] [PubMed]
- Breuss JM, Gillett N, Lu L, et al. Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. J Histochem Cytochem 1993;41:1521-7. [Crossref] [PubMed]
- AlDahlawi S, Eslami A, Hakkinen L, et al. The alphavbeta6 integrin plays a role in compromised epidermal wound healing. Wound Repair Regen 2006;14:289-97. [Crossref] [PubMed]
- Dietz HC. One integrin to rule them all? Sci Transl Med 2015;7. [Crossref] [PubMed]
- Hatley RJ, Macdonald SJ, Slack RJ, et al. An αv-RGD Integrin Inhibitor Toolbox: Drug Discovery Insight, Challenges and Opportunities. Angew Chem Int Ed Engl 2018;57:3298-321. [Crossref] [PubMed]
- Guo C, Wang Y, Liang H, et al. ADAMTS-1 contributes to the antifibrotic effect of captopril by accelerating the degradation of type I collagen in chronic viral myocarditis. Eur J Pharmacol 2010;629:104-10. [Crossref] [PubMed]
- Lee CW, Hwang I, Park CS, et al. Expression of ADAMTS-2, -3, -13, and -14 in culprit coronary lesions in patients with acute myocardial infarction or stable angina. J Thromb Thrombolysis 2012;33:362-70. [Crossref] [PubMed]
- Salter RC, Arnaoutakis K, Michael DR, et al. The expression of a disintegrin and metalloproteinase with thrombospondin motifs 4 in human macrophages is inhibited by the anti-atherogenic cytokine transforming growth factor-beta and requires Smads, p38 mitogen-activated protein kinase and c-Jun. Int J Biochem Cell Biol 2011;43:805-11. [Crossref] [PubMed]
- Wang X, Chen W, Zhang J, et al. Critical Role of ADAMTS2 (A Disintegrin and Metalloproteinase With Thrombospondin Motifs 2) in Cardiac Hypertrophy Induced by Pressure Overload. Hypertension 2017;69:1060-9. [Crossref] [PubMed]
- Colige A, Nuytinck L, Hausser I, et al. Novel types of mutation responsible for the dermatosparactic type of Ehlers-Danlos syndrome (Type VIIC) and common polymorphisms in the ADAMTS2 gene. J Invest Dermatol 2004;123:656-63. [Crossref] [PubMed]
- Dong C, Li HJ, Chang S, et al. A Disintegrin and Metalloprotease with Thrombospondin Motif 2 May Contribute to Cirrhosis in Humans through the Transforming Growth Factor-beta/SMAD Pathway. Gut Liver 2013;7:213-20. [Crossref] [PubMed]
- Bekhouche M, Leduc C, Dupont L, et al. Determination of the substrate repertoire of ADAMTS2, 3, and 14 significantly broadens their functions and identifies extracellular matrix organization and TGF-beta signaling as primary targets. FASEB J 2016;30:1741-56. [Crossref] [PubMed]
- Rurali E, Banterla F, Donadelli R, et al. ADAMTS13 Secretion and Residual Activity among Patients with Congenital Thrombotic Thrombocytopenic Purpura with and without Renal Impairment. Clin J Am Soc Nephrol 2015;10:2002-12. [Crossref] [PubMed]
- Rurali E, Noris M, Chianca A, et al. ADAMTS13 predicts renal and cardiovascular events in type 2 diabetic patients and response to therapy. Diabetes 2013;62:3599-609. [Crossref] [PubMed]
- Chion CK, Doggen CJ, Crawley JT, et al. ADAMTS13 and von Willebrand factor and the risk of myocardial infarction in men. Blood 2007;109:1998-2000. [Crossref] [PubMed]
- Kaikita K, Soejima K, Matsukawa M, et al. Reduced von Willebrand factor-cleaving protease (ADAMTS13) activity in acute myocardial infarction. J Thromb Haemost 2006;4:2490-3. [Crossref] [PubMed]
- Sonneveld MA, de Maat MP, Portegies ML, et al. Low ADAMTS13 activity is associated with an increased risk of ischemic stroke. Blood 2015;126:2739-46. [Crossref] [PubMed]
- Gombos T, Mako V, Cervenak L, et al. Levels of von Willebrand factor antigen and von Willebrand factor cleaving protease (ADAMTS13) activity predict clinical events in chronic heart failure. Thromb Haemost 2009;102:573-80. [Crossref] [PubMed]
- Witsch T, Martinod K, Sorvillo N, et al. Recombinant Human ADAMTS13 Treatment Improves Myocardial Remodeling and Functionality After Pressure Overload Injury in Mice. J Am Heart Assoc 2018.7. [PubMed]
- Scully M, Knobl P, Kentouche K, et al. Recombinant ADAMTS-13: first-in-human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood 2017;130:2055-63. [Crossref] [PubMed]
- Pehlivan S, Gurses MS, Ural MN, et al. The Role of ADAMTS1 and Versican in Human Myocardial Infarction: A Postmortem Study. Lab Med 2016;47:205-12. [Crossref] [PubMed]
- Jonsson-Rylander AC, Nilsson T, Fritsche-Danielson R, et al. Role of ADAMTS-1 in atherosclerosis: remodeling of carotid artery, immunohistochemistry, and proteolysis of versican. Arterioscler Thromb Vasc Biol 2005;25:180-5. [PubMed]
- Lind T, Birch MA, McKie N. Purification of an insect derived recombinant human ADAMTS-1 reveals novel gelatin (type I collagen) degrading activities. Mol Cell Biochem 2006;281:95-102. [Crossref] [PubMed]
- Lee NV, Sato M, Annis DS, et al. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. EMBO J 2006;25:5270-83. [Crossref] [PubMed]
- Luque A, Carpizo DR, Iruela-Arispe ML. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J Biol Chem 2003;278:23656-65. [Crossref] [PubMed]
- Zheng X, Chung D, Takayama TK, et al. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem 2001;276:41059-63. [Crossref] [PubMed]
- Xie Y, Li M, Wang X, et al. In vivo delivery of adenoviral vector containing interleukin-17 receptor a reduces cardiac remodeling and improves myocardial function in viral myocarditis leading to dilated cardiomyopathy. PLoS One 2013;8. [Crossref] [PubMed]
- Brilla CG, Rupp H, Maisch B. Effects of ACE inhibition versus non-ACE inhibitor antihypertensive treatment on myocardial fibrosis in patients with arterial hypertension. Retrospective analysis of 120 patients with left ventricular endomyocardial biopsies. Herz 2003;28:744-53. [Crossref] [PubMed]
- Vistnes M, Aronsen JM, Lunde IG, et al. Pentosan polysulfate decreases myocardial expression of the extracellular matrix enzyme ADAMTS4 and improves cardiac function in vivo in rats subjected to pressure overload by aortic banding. PLoS One 2014;9. [Crossref] [PubMed]
- Li J, Anemaet W, Diaz MA, et al. Knockout of ADAMTS5 does not eliminate cartilage aggrecanase activity but abrogates joint fibrosis and promotes cartilage aggrecan deposition in murine osteoarthritis models. J Orthop Res 2011;29:516-22. [Crossref] [PubMed]
- Velasco J, Li J, DiPietro L, et al. Adamts5 deletion blocks murine dermal repair through CD44-mediated aggrecan accumulation and modulation of transforming growth factor beta1 (TGFbeta1) signaling. J Biol Chem 2011;286:26016-27. [Crossref] [PubMed]
- Didangelos A, Mayr U, Monaco C, et al. Novel role of ADAMTS-5 protein in proteoglycan turnover and lipoprotein retention in atherosclerosis. J Biol Chem 2012;287:19341-5. [Crossref] [PubMed]
- Wang L, Zheng J, Bai X, et al. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ Res 2009;104:688-98. [Crossref] [PubMed]
- Kessler T, Zhang L, Liu Z, et al. ADAMTS-7 inhibits re-endothelialization of injured arteries and promotes vascular remodeling through cleavage of thrombospondin-1. Circulation 2015;131:1191-201. [Crossref] [PubMed]
- Pu X, Xiao Q, Kiechl S, et al. ADAMTS7 cleavage and vascular smooth muscle cell migration is affected by a coronary-artery-disease-associated variant. Am J Hum Genet 2013;92:366-74. [Crossref] [PubMed]
- Wu W, Zhou Y, Li Y, et al. Association between plasma ADAMTS-7 levels and ventricular remodeling in patients with acute myocardial infarction. Eur J Med Res 2015;20:27. [Crossref] [PubMed]
- Cain SA, Mularczyk EJ, Singh M, et al. ADAMTS-10 and -6 differentially regulate cell-cell junctions and focal adhesions. Sci Rep 2016;6:35956. [Crossref] [PubMed]
- Le Goff C, Morice-Picard F, Dagoneau N, et al. ADAMTSL2 mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-beta bioavailability regulation. Nat Genet 2008;40:1119-23. [Crossref] [PubMed]
- Tsutsui K, Manabe R, Yamada T, et al. ADAMTSL-6 is a novel extracellular matrix protein that binds to fibrillin-1 and promotes fibrillin-1 fibril formation. J Biol Chem 2010;285:4870-82. [Crossref] [PubMed]
- Neuhann TM, Stegerer A, Riess A, et al. ADAMTSL4-associated isolated ectopia lentis: Further patients, novel mutations and a detailed phenotype description. Am J Med Genet A 2015;167A:2376-81. [Crossref] [PubMed]
- Perrucci GL, Rurali E, Gowran A, et al. Vascular smooth muscle cells in Marfan syndrome aneurysm: the broken bricks in the aortic wall. Cell Mol Life Sci 2017;74:267-77. [Crossref] [PubMed]
- Hubmacher D, Apte SS. ADAMTS proteins as modulators of microfibril formation and function. Matrix Biol 2015;47:34-43. [Crossref] [PubMed]


