Approach to a solid solitary pulmonary nodule in two different settings—“Common is common, rare is rare”
Introduction
The differential diagnosis of a solid solitary pulmonary nodule (solid SPN) is broad, ranging from benign tumors, infectious lesions and lung cancer to other malignant conditions. Moreover, incidences vary widely in different parts of the world. While lung cancer is predominant in industrialized regions like Europe and North America, developing areas such as India, Central-, South- and West Africa as well as parts of South America have a high incidence of infectious lung diseases in the near absence of lung cancer: in North America and Europe where lung cancer affects 40-60/100,000 men and 13-22/100,000 women (1) 11,669 patients were newly diagnosed in 2010 in the Netherlands alone as opposed to only 189 patients with Tuberculosis (TB) in a previous year (2). In contrast South Africa experienced an almost 200 times higher population related incidence of 138,803 new cases of TB in 2008 (2). Conversely, the incidence of lung cancer in India and Central- and West-Africa is the lowest in the world with 0.9-10/100,000 men and 0.6-2.3/100,000 women (1).
Consequently, the approach to a solid SPN needs to happen in the context of geography and its respective socio-economic circumstances. To highlight the resulting diversity of diagnostic processes the approach to a new solid SPN is described in a setting such as Leeuwarden, the Netherlands and Cape Town, South Africa.
Definition SPN
A SPN is a common clinical problem. It may be accompanied by symptoms like cough, loss of weight, malaise or hemoptysis. In most instances the lesion is discovered incidentally on chest X-ray (CXR) or on computer tomography (CT) scan. Nowadays more and more lesions are discovered on CT scan, which is performed for various reasons like screening programs, investigations for pulmonary embolism or cardiac function and search for metastases of other cancers than lung cancer (3,4).
For this article the SPN will be defined as a single, spherical, well-circumscribed radiographic opacity of less than 3 cm in diameter with at least 2/3 of its margins surrounded by pulmonary parenchyma. Excluded in this definition are lymph nodes, atelectasis and post-obstructive pneumonia (5,6).
The major question that follows detection of a solid SPN is whether the SPN is malignant or not.
Prevalence of SPN
The detection rate of a SPN is 0.09% to 7% on routine chest radiographs (5). A study from the 1950’s showed that one of 500 CXRs demonstrated a SPN. In 1999 in the Early Lung Cancer Action Project, 7% of 1,000 healthy volunteers were found to have 1, 2 or 3 nodules in CXR screening (5). The failure to recognize lung cancer on the CXR is one of the most frequent causes of a missed diagnosis in radiology.
On CT scans the screening prevalence of at least one SPN is higher. It ranges there from 8% to 51% in the lung cancer screening trials using CT imaging (3,7-9). A positive screen result in the CT arm of the North American National Screening Trial (NLST) was defined as finding a non-calcified nodule of at least 4 mm. Using these criteria 27% had a positive baseline screen (9). The Netherlands-Belgium Lung cancer Screenings Trial (NELSON study) showed a rate of 51% non-calcified nodules at baseline (10).
Prevalence of malignancy in a SPN
As screening programs are typically performed in developed countries, the prevalence of malignancy in single pulmonary nodules reflects their incidences ranging from 1% to 12% in the various studies (11-13).
Differential diagnosis of SPN
As outlined, the differential diagnosis for a SPN is extensive (14,15). The frequency of each etiology varies amongst studies worldwide. Most studies were performed in the United States in specific cohorts. National data from Africa and Asia are scarce or missing. Tables 1,2 show examples from different settings, one from North America and one from Africa.

Full table
Full table
The distribution of the diagnoses in a newly found SPN is shown in Table 1, showing the wide spectrum in malignant and benign causes (15).
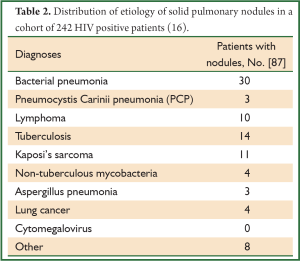
Another study reflects figures of a more common cohort in Africa [with a high incidence of human immunodeficiency virus (HIV)] and lists etiologies of pulmonary disease in 242 HIV infected patients undergoing a CT of the chest (Table 2) (16). In this cohort, 36% of patients had one or more pulmonary nodules. Four percent of patients had two concurrent diagnoses.
SPN evaluation
There are many approaches to evaluate a SPN. Significant variation exists among institutions influenced by the socio-economic and inherent circumstances of a region (5,14,15,17-21).
We will try to outline the approaches in a setting of high prevalence of lung cancer and a low incidence of infectious diseases, like North America or Europe in contrast to areas with a high incidence of TB, HIV and other infectious diseases and a low incidence of lung cancer. For this article we will limit ourselves to the solid SPN, and leave the field of ground glass nodules and semisolid/subsolid SPN for a discussion elsewhere.
The ideal approach to a solid SPN should result in the resection of all malignant nodules, while avoiding resection of all benign nodules that require no treatment or some form of medical treatment.
Setting 1: SPN evaluation in Leeuwarden, the Netherlands
Patients with a newly diagnosed solid SPN undergo an initial diagnostic evaluation based on radiological findings and medical, often interdisciplinary judgment to determine the probability of being a malignant nodule. In this setting this is the most important question as the patients with stage 1A (T1N0M0) lung carcinoma have the best prognosis and the highest potential for cancer cure (1). We follow the guidelines “The Solitary Pulmonary Nodule” published in Chest 2003 by the ACCP and the next version published in Chest in 2007 by Patel and coworkers (5,14,15,19).
The following indices give us guidance in the approach to a solid SPN.
Clinical characteristics
Older age, a history of cigarette smoking, larger nodule size, female gender, asbestos exposure and previous history of cancer all increase the probability that a solid SPN is malignant (22). The presence of underlying lung disease such as emphysema, fibrotic lung disease (idiopathic pulmonary fibrosis, radiation and asbestosis) are additional risk factors (23).
Radiologic features
Chest radiograph (CXR)
There are no characteristics that can consistently distinguish between a benign nodule and a malignant nodule based on the appearance on CXR. However a solid SPN that is unchanged for more than two years is unlikely to be a malignant nodule. A CXR is less sensitive than a CT scan for detecting changes in size, density and borders of a solid SPN. The first step is to compare the lesion with previous CXRs or CT scans. If that is not available the next step is a high resolution CT scan.
Chest computed tomography (CT)
CT is more sensitive and more specific than CXR for detecting nodules (23). Additionally, the CT detects intra-thoracic abnormalities like enlarged lymph nodes or tumors in the mediastinum or other blind spots (hidden by diaphragm, heart or bony structures).
Features that can be used to predict whether a nodule is malignant include size, border, calcification, density, growth and location (24):
- Size: larger lesions are more likely to be malignant than smaller lesions. Lesions >3 cm in diameter should be considered malignant until proven otherwise (25,26):
- Nodules ≤3 mm: 0.2% likelihood of malignancy;
- Nodules 4-7 mm: 0.9% likelihood of malignancy;
- Nodules 8-20 mm: 18% likelihood of malignancy;
- Nodules >20 mm: 50% likelihood of malignancy.
- Border: malignant lesions tend to have a more irregular, lobulated and spiculated border, whereas benign lesions often have a relatively smooth and discrete border (27) (Figures 1,2). The presence of small satellite nodules surrounding a dominant pulmonary nodule is characteristic of a benign nodule, typically a granuloma.
- Calcification: calcification is highly suggestive of a benign lesion, especially when it has an organized pattern (central, laminated or popcorn pattern).
- Density: the attenuation of a nodule (Hounsfield Units = HU) is a reflection of the amount of calcium within the nodule. The presence of focal fat within a solid SPN is highly suggestive of a benign solid SPN like a hamartoma.
- Cavitation: thin walled cavitating nodules are more likely to be benign. A thick walled cavity (>15 mm) is a feature of a squamous cell carcinoma, but is also present in many TB cases or aspergillomas.
- Growth rate: the doubling time for the majority of malignant lesions is 30-480 days (28). The exception of this rule includes slow growing tumors such as adenocarcinoma in situ (broncho-alveolar carcinoma in the old classification) with a doubling time up to 900 days, which is often ground glass in appearance.
- All malignant, fast growing lung nodules followed up from the baseline CT scan after three months in the NELSON trial (multinational screening trial) had a volume-doubling time less than 232 days (29).
- Location: malignant lesions are more likely to be in the upper lobes, but the same applies to TB and aspergillomas.

Probability tests
The most frequently used model for assessing the risk of malignancy is the Bayesian analysis, which postulates that the post test probability of disease is linked to pre test probability and the sensitivity and specificity of the test (19). The method is based on estimating the probability of being a malignancy using nodule size, presence of spiculae and location, patients age, smoking history and previous malignancies (30,31). The overall prevalence of malignancy in the population is also important. A recent study shows that the inclusion of nodule volume in the malignancy prediction model increases the proportion of nodules correctly classified (26,32).
Management
The algorithm outlined in Figure 3 is guiding the diagnostic and management decisions [including the probability test (15)] in the approach to a solid SPN. All decisions get based on a multidisciplinary panel consensus that takes the patient’s preference into consideration. As such, a patient will fall into one of three groups: High (>60%), intermediate (5-60%) and low (<5%) risk for developing lung cancer.
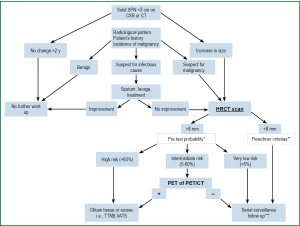
- A solid SPN that remains stable for more than two years can be considered benign acknowledging that certain low grade adenocarcinomas and carcinoids can be stable for two years or longer.
- A nodule that grows or a nodule in a patient with a high risk probability (>60%) should be biopsied or excised (Figure 1) unless the patient is not fit for such an intervention or refuses it.
- A solid SPN smaller than 8 mm can be followed up by using the guidelines proposed by the Fleischner Society (25). In 2005 the Fleischner Society guidelines for managing pulmonary nodules detected on CT scans were developed in response to a perceived need for managing incidental small nodules detected on CT scans (Table 3).
- In a solid SPN with an intermediate risk probability a 18F-Fluorodeoxyglucose positron emission tomography (FDG-PET) is recommended.

Full table
Positron emission tomography (PET) CT
It is estimated that 96% of patients with lung cancer will have an abnormal FDG-PET and 78% of patients without lung cancer will have a normal FDG-PET (33-35). PET has a very high sensitivity (89-100%), but a lesser specificity (69-100%) and an accuracy of 89-96% in a modern combined modality PET/CT (36-38). PET is false positive in granulomatous disease and is usually false negative in lesions of less than 0.8 cm (5,39). Other pitfalls of PET are false negative findings in metabolically low-activity tumors (adenocarcinoma in situ or minimally invasive mucinous adenocarcinoma and carcinoid tumors) and in hyperglycaemia (39).
In intermediate risk patients a PET scan is performed as the next diagnostic step. In case of PET negativity the lesion will be followed up after 3, 6, 12 and 24 months with a low dose CT in line with the recommendations of The American College of Chest Physicians (15).
When the PET scan is positive the probability of malignancy is high in our setting. The following figures are two examples from our daily practice representing newly found solid SNPs. Treatment follows our algorithm and gets individualized according to the patient’s condition and preferences.
The first case is a PET positive lesion in a 54-year-old heavy smoker with chronic obstructive airway disease GOLD II. He underwent a PET scan according to our algorithm as a medium risk patient (Figure 4), followed by a transthoracic needle biopsie (TTNB) (squamous cell carcinoma) and a VATS lobectomy with systematic lymph node dissection (pT1N0M0).
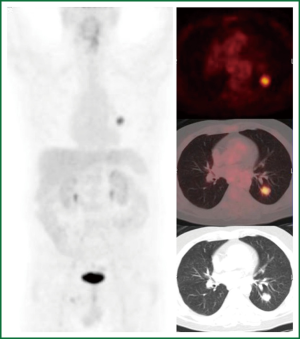
The second case is a 63-year-old ex-smoker (stopped 30 years ago) with a history of a renal cell adenocarcinoma (pT3aN0M0) treated by nephrectomy six years ago, poor left ventricular function and a coronary bypass graft operation in 2012. At the preoperative work up in 2012 a new lung lesion was found. Again according to our algorithm a PET scan was performed (Figure 5). This patient was followed up for three months. On repeat CT scan the lesion had grown. Differential diagnosis included a benign lesion, a metastasis from the renal cell cancer or a PET negative lung cancer such as a carcinoid or some forms of adenocarcinoma. TTNB got some malignant cells compatible with a metastasis from the previous renal cell (Grawitz) tumor. Due to the overall condition of this patient stereotactic radiation was performed.
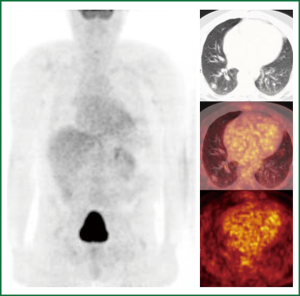
A systematic review concludes that the additional information gained from PET imaging in diagnosing a malignant SPN is worth the costs providing good medical indications are applied (40). Implementation of the PET-CT as a combined modality is variable, depending on the health care system and the uncertainty about its cost-effectiveness. In our institution this will become standard next year, at present CT and PET are two machines and get combined by a computer program.
Tissue diagnosis and therapeutic approach
A management decision should be made once the probability that the SPN is malignant or likely to be malignant has been assessed (see algorithm in Figure 3). For high risk patients or PET positive lesions it is mandatory to get a tissue diagnosis but how to obtain the tissue is under debate. There is a lack of published data directly comparing the results from biopsies obtained using different methods. The location of the nodule and the likelihood of complications determine which approach is used. Preferences and expertise of the institution seem to play a role (41).
- Bronchoscopy can be used to evaluate larger or more central nodules (42). However it is much less useful for small (<2 cm) or peripheral solid SPNs.
- Fluoroscopy or endobronchial ultrasound (EBUS) can optimize localization of the solid SPN.
- TTNB: the procedure is reported to have a sensitivity of 64-100% (14). Adding core needle biopsy to the procedure is more likely to establish the diagnosis especially for non-malignant lesions (14,43). A positive result is a reliable indicator of malignancy but a negative result is of limited value in excluding malignancy. Complications are hemorrhage and pneumothorax. In studies of CT guided needle biopsies, non-diagnostic results were seen in 20%.
- Surgery: surgical resection of the solid SPN by thoracotomy or video assisted thoracoscopic surgery (VATS) is common practice for a growing solid SPN, a solid SPN with a high likelihood of malignancy or a proven malignant solid SPN. In combination with a frozen section, the definitive treatment can be achieved in the same session. Whether a wedge resection or a VATS lobectomy with systematic lymph node dissection is necessary is determined by the results of the frozen section. In rare cases the frozen section of the VATS wedge resection cannot differentiate between a metastasis and a primary lung tumor. In these circumstances a staged approach after final histology including immunohistochemistry is adopted. Radio-guided surgery is the preferable method to locate a sub-centimeter solid SPN and mark it with a wire in order to achieve a successful excision instead of finger palpation (44). Intra-operative ultra-sonography is not available in Leeuwarden.
Setting 2: SPN evaluation in South Africa, Cape Town
The approach to a new solid SPN in endemic areas of infectious pulmonary disease like TB, HIV related disorders (Pneumocystis jiroveci, bacterial pneumonia, Kaposi etc.) and fungal disease is different. The standard algorithms published for setting 1 (19) need to be modified especially in the first phase. This will be outlined in the following chapter.
Clinical characteristics
In these endemic areas of TB and HIV infection the patients’ history, symptoms and contact history play a significant role in the diagnostic and therapeutic approach.
HIV positive patients with a CD4 count >200 are treated as any other patient. With lower CD4 counts, however, more emphasis will be put on the search for pulmonary infections. In a study by Jasmer and coworkers 36% of the HIV patients had one or more pulmonary nodules on chest CT. Multivariate analysis identified fever, cough, and a nodule size of <1 cm on chest CT as independent predictors of having an opportunistic infection. Furthermore, a history of bacterial pneumonia, symptoms for one to seven days, and a nodule size of <1 cm on CT independently predicted the diagnosis of bacterial pneumonia. A history of homelessness, weight loss, and lymphadenopathy on CT independently predicted a diagnosis of TB (16).
Search for an infective source
The search for an infective source will include induced sputum, bronchoscopy and lavage and in indicated cases (i.e., for enlarged lymph nodes) EBUS /EUS biopsies. With an incidence of TB being as hjgh as 981/100,000, a new lesion warrants active search for acid fast bacilli (AFB). Sensitivities and specificities for induced sputum smear microscopy lie in the range from 13-40% and 90-99%, respectively (6,45,46). Diagnostic progress was made in the last few years by introducing diagnostic rapid TB tests (e.g., the Xpert® assay). In recent studies the sensitivity of the Xpert® assay using bronchial washings or broncho-alveolar lavage fluid for the diagnosis of pulmonary TB was 81-96%, and the specificity was 98-100% (47-49). The positive predicted value and the negative predicted value were 100% and 92.1%, respectively (49).
Management
Results from TB cultures are not available for at least six weeks.
- In cases highly suspicious for TB with typical symptoms, positive contacts, younger age, HIV infection etc. empiric TB treatment is started. These patients need to be followed up closely and seen again within six weeks for reassessment of their symptoms. A repeat CXR is obtained to assess the growth pattern of the lesion. In this situation the suggested algorithm (Figure 3) can be followed to the left without a CT scan. Standard anti-TB treatment gets started, culture results obtained and a CXR taken after three and six months to monitor the effect of the applied treatment. In addition, the symptomatology of the patient is very important to ascertain whether there is improvement on anti-TB treatment. Targeted treatment is applied once the sensitivities are known. This approach is also supported by the WHO-TB guidelines (2). In cases of multi-drug resistant TB or a mycobacterium other than Tuberculosis (MOTT) the lesion will not change and treatment needs to be adapted.
- In cases of less obvious clinical suspicion for TB or other infectious diseases a high resolution CT scan serves as a basis to gain more information over the exact location, the density and borders of the lesion, over lymph nodes, lung parenchyma or additional small nodules other than the solid SPN seen on CXR.
The following two cases represent our daily practice.
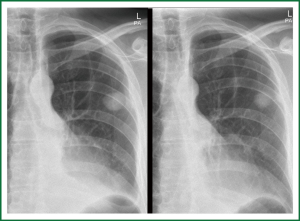
The first case is a 40-year-old black male presenting with a productive cough, mild loss of weight and general malaise. He was HIV negative and had no TB contacts. CXR revealed a SPN in his left upper lobe. Induced sputum samples on three consecutive mornings were negative, bronchoscopic lavage results only showed mixed organism, TTNB revealed the presence of AFB’s and standard anti-TB treatment was started (Figure 6).
The second case is a 65-year-old white male, heavy smoker, HIV negative, pacemaker dependant presenting with a new SPN on routine CXR. CT scan revealed a well circumscribed lesion in his right middle lobe. Bronchoscopic biopsies showed inflammatory cells, no malignancy and cultures showed mixed organism, no AFB’s, TB cultures awaiting. He also went for TTNB to establish diagnosis falling into the intermediate risk group on calculations (Figure 7).
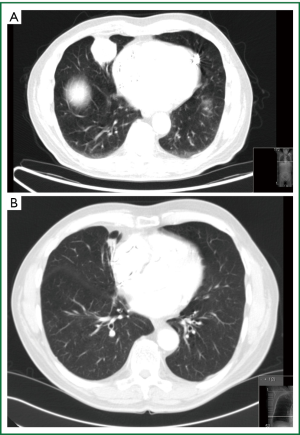
If there is a strong probability for the lesion to be a malignant lesion, as previously described, a tissue diagnosis of the lesion is being obtained. Tissue diagnosis is obtained by means of TTNB, VATS wedge or open wedge to distinguish malignancy from inflammatory disease. TTNB is the preferred investigation in our institution as we have a very experienced radiological team and the majority of lesions in our daily practice turn out to be infectious of nature (Figures 6-8). Early tissue diagnosis helps us to plan the further work up and treatment options.
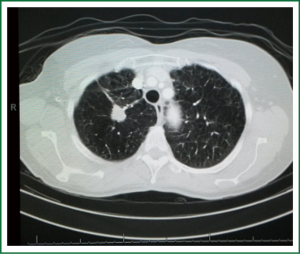
If the diagnosis confirms malignancy the staging of the tumor follows established guidelines (50). Enlarged lymph nodes will be biopsied (via EBUS biopsy, mediastinoscopy or via a VATS approach) in order to stage the tumor.
The PET scan
The PET scan is useful for staging a proven malignant lesion, also in this setting but is not widely available.
In the initial work up to establish a diagnosis and in order to distinguish malignancy from infectious or inflammatory disease the PET scan is less helpful as increased FDG activity on PET scan is found in all three conditions. For the initial assessment of the solid SPN we do not use this modality due to the high incidence of false positive findings with infections and inflammation.
A multi-disciplinary team, including a thoracic surgeon, a respiratory physician and a radiologist discuss the steps outlined above routinely in our own institution as well as in its affiliated satellite hospitals. The area served is spread out over the entire Western Province, with some hospitals being more than 600 km away. This is achieved by regular visits or teleconference. If any doubt exists amongst the treating physicians, the patients are referred to our centre.
Discussion
A solid SPN newly diagnosed on routine CXR or on (screening) CT is a common problem. We describe the decision making process in the Netherlands and in South Africa, a country with a high incidence of TB and other inflammatory diseases. It is clear that the general statement: “a new nodule in a smoker aged over 50 is a primary lung cancer until otherwise proven” is too simplistic to be used all over the world.
The differential diagnosis is wide. One needs to know the incidences of different etiologies (malignant, infectious and inflammatory disease) of a solid SPN in the area the patient comes from. In setting 2 a new pulmonary nodule is often regarded as TB until otherwise proven, especially in HIV positive patients. Incidence of lung cancer in Africa is very low, partly due the lower median life expectancy in developing countries. People die earlier from other diseases before lung cancer can develop. Hereditary factors may also play a role as well as different smoking habits. Unfortunately exact incidence and prevalence data are not widely available for developing countries (1,2), the most reliable information coming from the World Health Organization: www.who.int. Yet, the general rule: “common is common, rare is rare” applies.
In developed countries more insight in these data is available, also due to data from big screening trials where prevalence, follow up, treatment and outcome data is well recorded. The North American National Lung Screening Trial (NLST) is by far the largest series. So far, the NLST study is the only randomized controlled trial that has shown mortality reduction by using CT screening (10,51). Problems identified with CT screening include false positive, benign nodule resections, over-diagnosis, the exposure to radiation and costs (38,52,53). An estimated expense of 50,000 Euro per life year gained was reported (1,54). In the Netherlands, the prospective randomized NELSON screening trial did not show such a mortality reduction and the discussion is ongoing (12,45,54-58).
For follow up of a solid SPN <8 mm, the Fleischner Society guidelines are used in a flexible manner. Strict adherence to the Fleischner Society guidelines for managing pulmonary nodules detected on CT scans is questionable and it does not seem to have found general acceptance. A review article from 2012 showed that the radiologists in a Large Community Hospital do not always adhere to the Fleischner criteria. In most cases they recommend a closer follow-up (59). We find the same in our clinical practices.
In the evaluation of a new solid SPN we believe that the PET scan is an extremely helpful tool besides its established role in the staging for lung cancer and differentiating it from metastatic disease. It is a cornerstone under the conditions of a developed country as outlined in the algorithm. Not using a PET scan in South Africa has various reasons from limited accessibility of tertiary health care facilities to budget constraints. Another reason is the lack of specificity in glucose uptake to distinguish inflammatory disease from cancer (33,34,36). Therefore, its predicting power for malignancy is limited for such a setting. As such, the value of performing a PET scan as outlined in the algorithm in ‘setting 2’ is questionable and as a consequence, this step is often skipped. For patients with intermediate risk it very much depends on the individual case. Diagnostic steps are focused on obtaining a tissue diagnosis i.e., TTNB or wedge resection (see Figures 6,7). PET scan (where available) is advisable in the further work up of the patient with a proven malignancy.
The use of calculators for predicting the risk of malignancy is also a very helpful tool (22,30,32). However, one has to take into consideration that the development of such calculators (probability tests) was based on cohorts in countries with high incidence of lung cancer and low incidence of infectious diseases. While they are in principle applicable in all settings, conclusions for countries such as South Africa, India and China should be less strict as TB can mimic the features of lung cancer (Figure 8). Upper lobes are the predilected location for both TB and aspergillomas.
In general, the algorithms available and the risk-calculating methods do help in the decision making process. Physician, nuclear physician, radiologist and surgeon need to judge the probability of a malignancy. The patient and the health authorities play an increasingly important role in the choice of investigations and therapies.
One cannot point out strongly enough that the key issue in a new solid SPN is to establish a diagnosis for the surgical resection of early stage lung cancer. Stage I non small cell lung cancer (NSCLC) has a 5-year survival rate of approximately 60-80% (1). Whether this is achieved via a TTNB or directly via the surgeon, who will first perform a wedge resection and in the same session a lobectomy as the SPN turns out to be malignant, is handled differently in different institutions, mainly dependent on the expertise available in a center and individual preferences. Minimal invasive techniques are commonly used nowadays and therefore one tends to prefer an established diagnosis on histology rather than relying on frozen section. CT guided biopsies are commonly used with a good diagnostic yield. In case of functional inoperability stereotactic radiation is a good alternative (60,61).
In summary, the evaluation process for a new solid SPN in the two different areas—the Netherlands and South Africa—follows the same principles. Yet, the initial diagnostic steps differ because of the different incidence of the various etiologies of a solid SPN in these two areas. The proposed algorithm is a valuable tool for the teams in day-to-day practice, especially in challenging cases. It provides a framework incorporating many sets of guidelines for the different scenarios also in different countries. There is general consensus that management should be individualized and carefully adapted to the patient’s condition and preferences.
The approach to the solid SPN will probably modify with evolving technologies like volumetric imaging and advanced bronchoscopic techniques. Cost-effectiveness will also play an even more dominant role in the future (40,42,62).
Conclusions
To evaluate a solid SPN the physician needs to know what incidences and disease burdens are present in his/her area. Probabilities and risk stratification of being a malignant solid SPN needs to be judged accordingly. Models, calculators and guidelines are helpful tools for the countries with a high incidence of lung cancer.
Despite the difficulties in advising a general algorithm we believe that the stepwise approach outlined above is helpful in the decision making process in the developed and developing world. The team of physicians can use it as a template to provide individualized treatment in a newly diagnosed solid SPN.
Acknowledgements
The authors thank the Department of Radiology and the Department of Nuclear Medicine, Medical Center Leeuwarden for their great contribution regarding the figures.
Disclosure: The authors declare no conflict of interest.
References
- Huber RM. Manual Tumoren der Lunge und des Mediastinum. Muenchen: W. Zuckerschwerdt Verlag, 2011.
- World Health Organization. Available online: www.who.int, 2013
- Veronesi G, Maisonneuve P, De Pas TM, et al. Does lung cancer screening with low-dose CT remain promising despite disappointing DANTE results? Am J Respir Crit Care Med 2010;182:720-1; author reply 721. [PubMed]
- Veronesi G, Maisonneuve P, Spaggiari L, et al. Long-term outcomes of a pilot CT screening for lung cancer. Ecancermedicalscience 2010;4:186. [PubMed]
- Patel VK, Naik SK, Naidich DP, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 1: radiologic characteristics and imaging modalities. Chest 2013;143:825-39. [PubMed]
- Tuddenham WJ. Glossary of terms for thoracic radiology: recommendations of the Nomenclature Committee of the Fleischner Society. AJR Am J Roentgenol 1984;143:509-17. [PubMed]
- Henschke CI, Yankelevitz DF, Naidich DP, et al. CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans. Radiology 2004;231:164-8. [PubMed]
- Gohagan J, Marcus P, Fagerstrom R, et al. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the Lung Screening Study of the National Cancer Institute. Chest 2004;126:114-21. [PubMed]
- Diederich S, Wormanns D, Heindel W. Radiologic screening for lung cancer: present status and future perspectives. Rofo 2001;173:873-82. [PubMed]
- Ru Zhao Y, Xie X, de Koning HJ, et al. NELSON lung cancer screening study. Cancer Imaging 2011;11 Spec No A:S79-84.
- Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:94S-107S.
- Midthun DE. Early diagnosis of lung cancer. F1000Prime Rep 2013;5:12. [PubMed]
- Henschke CI, Yankelevitz DF, Naidich DP, et al. CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans. Radiology 2004;231:164-8. [PubMed]
- Tan BB, Flaherty KR, Kazerooni EA, et al. The solitary pulmonary nodule. Chest 2003;123:89S-96S. [PubMed]
- Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:108S-30S.
- Jasmer RM, Edinburgh KJ, Thompson A, et al. Clinical and radiographic predictors of the etiology of pulmonary nodules in HIV-infected patients. Chest 2000;117:1023-30. [PubMed]
- Schiavon F, Berletti R, Soardi GA, et al. Multidisciplinary management of the solitary pulmonary nodule (SPN): our opinion. Radiol Med 2005;110:149-55. [PubMed]
- Romero Candeira S, Alemany Frances L, Martin Serrano C, et al. The solitary pulmonary nodule. An analysis of the experience over 6 years in a medical service. An Med Interna 1994;11:427-30. [PubMed]
- Patel VK, Naik SK, Naidich DP, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 2: pretest probability and algorithm. Chest 2013;143:840-6. [PubMed]
- Alzahouri K, Velten M, Arveux P, et al. Management of SPN in France. Pathways for definitive diagnosis of solitary pulmonary nodule: a multicentre study in 18 French districts. BMC Cancer 2008;8:93. [PubMed]
- Dabrowska M, Kolasa A, Zukowska M, et al. Analysis of solitary pulmonary nodules found in chest radiograms. Pneumonol Alergol Pol 2009;77:37-42. [PubMed]
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. [PubMed]
- Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920-31. [PubMed]
- Xu DM, Gietema H, de Koning H, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006;54:177-84. [PubMed]
- MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395-400. [PubMed]
- Mehta HJ, Ravenel JG, Shaftman SR, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest 2014;145:464-72. [PubMed]
- Marten K, Engelke C, Seyfarth T, Grillhosl A, et al. Computer-aided detection of pulmonary nodules: influence of nodule characteristics on detection performance. Clin Radiol 2005;60:196-206. [PubMed]
- Henschke CI, Yankelevitz DF, Yip R, et al. Lung cancers diagnosed at annual CT screening: volume doubling times. Radiology 2012;263:578-83. [PubMed]
- Heuvelmans MA, Oudkerk M, de Bock GH, et al. Optimisation of volume-doubling time cutoff for fast-growing lung nodules in CT lung cancer screening reduces false-positive referrals. Eur Radiol 2013;23:1836-45. [PubMed]
- Swensen SJ, Silverstein MD, Ilstrup DM, et al. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997;157:849-55. [PubMed]
- Gould MK, Ananth L, Barnett PG, Veterans Affairs SNAP Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest 2007;131:383-8. [PubMed]
- Wang Y, de Bock GH, van Klaveren RJ, et al. Volumetric measurement of pulmonary nodules at low-dose chest CT: effect of reconstruction setting on measurement variability. Eur Radiol 2010;20:1180-7. [PubMed]
- Vansteenkiste JF. Imaging in lung cancer: positron emission tomography scan. Eur Respir J Suppl 2002;35:49s-60s. [PubMed]
- Vansteenkiste JF, Stroobants SS. PET scan in lung cancer: current recommendations and innovation. J Thorac Oncol 2006;1:71-3. [PubMed]
- Barger RL Jr, Nandalur KR. Diagnostic performance of dual-time 18F-FDG PET in the diagnosis of pulmonary nodules: a meta-analysis. Acad Radiol 2012;19:153-8. [PubMed]
- Jeong YJ, Yi CA, Lee KS. Solitary pulmonary nodules: detection, characterization, and guidance for further diagnostic workup and treatment. AJR Am J Roentgenol 2007;188:57-68. [PubMed]
- Gould MK, Maclean CC, Kuschner WG, et al. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 2001;285:914-24. [PubMed]
- Buck AK, Herrmann K, Stargardt T, et al. Economic evaluation of PET and PET/CT in oncology: evidence and methodologic approaches. J Nucl Med Technol 2010;38:6-17. [PubMed]
- Chest-Lung-Solitary pulmonary nodule: benign versus malignant differentiation with CT and PET-CT January 2013. Radiological Society of the Netherlands. Available online: www.radiologyassistant.nl (Accessed July/29, 2013) .
- Cao JQ, Rodrigues GB, Louie AV, et al. Systematic review of the cost-effectiveness of positron-emission tomography in staging of non-small-cell lung cancer and management of solitary pulmonary nodules. Clin Lung Cancer 2012;13:161-70. [PubMed]
- Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med 2012;185:363-72. [PubMed]
- Ray CE Jr, Mohammed TL. Review of ACR Appropriateness Criteria® Radiologic Management of Thoracic Nodules and Masses. J Thorac Imaging 2012;27:W85-6. [PubMed]
- Rivera MP, Mehta AC, American College of Chest Physicians. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:131S-48S.
- Zaman M, Bilal H, Woo CY, et al. In patients undergoing video-assisted thoracoscopic surgery excision, what is the best way to locate a subcentimetre solitary pulmonary nodule in order to achieve successful excision? Interact Cardiovasc Thorac Surg 2012;15:266-72. [PubMed]
- Wender R, Fontham ET, Barrera E Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin 2013;63:107-17. [PubMed]
- Available online: http://www.who.int/tb/publications/2009/who_htm_tb_2009_420_beforeprint.pdf
- Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011;377:1495-505. [PubMed]
- Lawn SD, Mwaba P, Bates M, et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis 2013;13:349-61. [PubMed]
- Lee HY, Seong MW, Park SS, et al. Diagnostic accuracy of Xpert(R) MTB/RIF on bronchoscopy specimens in patients with suspected pulmonary tuberculosis. Int J Tuberc Lung Dis 2013;17:917-21. [PubMed]
- De Leyn P, Lardinois D, Van Schil PE, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;32:1-8. [PubMed]
- International Early Lung Cancer Action Program Investigators, Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Berg CD, et al. The National Lung Screening Trial: overview and study design. Radiology 2011;258:243-53. [PubMed]
-
National Lung Screening Trial Research Team, Church TR, Black WC,
et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [PubMed] - Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. [PubMed]
- Prosch H, Strasser G, Oschatz E, et al. Management of patients with small pulmonary nodules: a survey of radiologists, pulmonologists, and thoracic surgeons. AJR Am J Roentgenol 2006;187:143-8. [PubMed]
- Diederich S. Lung cancer screening: status in 2007. Radiologe 2008;48:39-44. [PubMed]
- Nair A, Hansell DM. European and North American lung cancer screening experience and implications for pulmonary nodule management. Eur Radiol 2011;21:2445-54. [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [PubMed]
- Masciocchi M, Wagner B, Lloyd B. Quality review: Fleischner criteria adherence by radiologists in a large community hospital. J Am Coll Radiol 2012;9:336-9. [PubMed]
- Guckenberger M, Allgauer M, Appold S, et al. Safety and efficacy of stereotactic body radiotherapy for stage i non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysis. J Thorac Oncol 2013;8:1050-8. [PubMed]
- Palma D, Lagerwaard F, Rodrigues G, et al. Curative treatment of Stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys 2012;82:1149-56. [PubMed]
- van Klaveren RJ, Oudkerk M, Mali WP, et al. Multi-detector CT screening for lung cancer is still to be discouraged for the time being. Ned Tijdschr Geneeskd 2008;152:125-8. [PubMed]


