A simple patient-tailored aortic arch tangential angle measuring method to achieve better clinical results for thoracic endovascular repair of type B aortic dissection
Introduction
Acute type B aortic dissection (AD), mainly involving the descending aorta, is still a life-threatening condition with high mortality and morbidity and remains a formidable challenge for either cardiac or vascular surgeons (1). During the past decade, endovascular techniques have revolutionized the management of descending thoracic aortic disease, with the benefit of exclusion of the lesion without direct surgical exposure (2). However, thoracic endovascular repair (TEVAR) procedure requires the lesion to fulfill several basic anatomical criterions. The essential requirement is to have an enough proximal landing zone (PLZ) longer than 15 mm (3,4). Unfortunately, a large proportion of type B AD patients have the proximal tear located near the orifice of the left subclavian artery (LSA), which render these lesions anatomically unsuitable for routine TEAVR. Additional procedures like carotid to carotid bypass and chimney are required to extend PLZ or to preserve the supra-aortic branch. From craniocaudally orientated cross-section view, the aortic arch extent from ascending aorta along a left-posterior direction into descending one. The aortic arch tangential angle (θ-AATA) is a crucial anatomical parameter in terms of measuring during TEVAR. Generally, this angle is estimated approximately as 45 left-anterior oblique (LAO) degrees (5). During daily practice, the length of PLZ was measured and documented based on the aortic arch angiography, which was conducted routinely at LAO-45 degrees. The projection of PLZ could achieve its maximal length when the beam of X-ray is perpendicular to aortic arch. Applying an exact tangential angle angiography of aortic arch is essential to get the longest PLZ for better case planning, more precise deployment, and therefore a better outcome. Recently, an individualized patient tailored specific measuring method was adopted in our center to obtain the exact θ-AATA. The aim of this study is to introduce this new angle measuring method and report its preliminary effect on the clinical results.
Methods
Study design
The study was approved by institutional ethics committee of Zhongshan Hospital (No. 2017-204). Written informed consent was obtained from each patient. From January 2013 to December 2014, the patients fulfilled the inclusion criterion were enrolled in this study. Inclusion criterions consist of: (I) patients were diagnosed as type B AD with contrasted CT scan; (II) the proximal tear is close to the ostium of LSA; (III) patients consent for TEVAR treatment; (IV) patients have definitive indications for TEVAR. The indications for TEVAR treatment in this study are as same as the widely accepted one: mal-perfusion syndrome, rapidly enlargement of the false lumen aneurysm (≥5.5 cm), pending rupture, refractory hypertension and refractory pain (6). Exclusion criterions include: (I) patients have any hereditary genetic diseases like Mafan syndrome; (II) patients have previous open thoracic aortic surgery history; (III) patients have any previous thoracic aortic endovascular therapy history.
Angle measurement
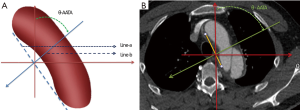
The measurement was based on the axial contrast enhanced CT scan (128 slices, slice thickness 0.5 mm, increment 0.5 mm). Briefly, draw a line (line-a) parallel to the aortic arch and another line (line-b) perpendicular to line-a was drawn accordingly. Then, the angle between line-b and the anterior-posterior line was measured and recorded. This angle was defined as the θ-AATA (Figure 1). Each patient was measured by two different physicians to ensure that the inter-observer difference was within 15% threshold.
Procedure
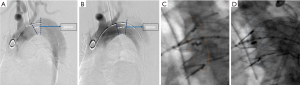
The entire TEVAR procedure of this study was almost same as the routinely carried out one except the initial aortic arch angiography to measure PLZ and the final fluoroscopy-to determine the proximal marks’ alignment. The detailed procedure is briefly described as below. Firstly, perform a routine LAO-45 degrees’ aortic arch angiography and measure the length of PLZ (L1) (Figure 2A). Secondly, conduct another LAO angiography at θ-AATA and measure [with the build-in measuring method of the digital subtraction angiography (DSA) work-station] the PLZ length (L2) (Figure 2B). Next, fix the DSA bed and C-arm, mark all the supra arch branch ostium, confirm the position at which the proximal edge of the stent-graft supposed to be deployed and forward the stent-graft to this position. Then, the stent-graft was deployed in a standard fashion. Take another aortic arch angiography to check the efficacy of sealing and detect the occurrence of type I endoleak. Finally, evaluate the alignment of proximal marks of the deployed stent-graft at the routine LAO-45 degrees’ angle and at θ-AATA (Figure 2C,D). All patients were scheduled for follow-up assessment, including routine office visits and contrast CT scan at 1, 6, and 12 months after stent-graft implantation and annually thereafter.
Study endpoints and definition
The primary endpoints of this study were the measured length of PLZ and the alignment of the stent-graft’s proximal marks based on these two different LAO angles. The secondary endpoints included the incidence of the supra-aortic arteries’ mis-coverage and the immediate type I endoleak. Besides these endpoints, the data including the measured θ-AATA and the stent-graft’s deployment strategy was also individually recorded.
Statistical analysis
Categorical data (such as the number of patients need to cover LSA and the number of the patients with stent-graft reach proximal marks’ alignment) are described using absolute numbers, whereas continuous variables (such as age, angle and length of PLZ) are presented as mean ± standard deviation. The threshold of statistical significance was P<0.05. The statistical analysis was performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC, USA).
Results
Periprocedural patient characteristics
A total of 76 patients were enrolled in this study. Among them, 78.9% was male and the mean age was 59.8 years old (range, 36–82 years). Table 1 outlined all the clinical features of the study cohort. The average time from symptom onset to diagnosis was 1.2 days (range, 0–9 days), with 98% of the patients appropriately diagnosed within one week of symptom onset. All the patients were treated in acute stage. The mean time period from onset to TEVAR treatment was 12.3 days (range, 3–15 days).
Full table
Measured data
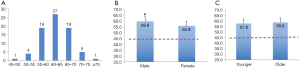
The average value of θ-AATA was 58.3±5.2 degrees, which was significantly larger than the generally applied LAO-45 degrees (P<0.01), and most of the patients’ θ-AATA were in the 55–70 degrees’ range (Figure 3A). The mean θ-AATA in male group (59.8±5.5 degrees) is larger than that in female group (55.9±3.8 degrees) (P<0.05), and both of them are greater than 45 degrees (P<0.01) (Figure 3B). There is no difference between the older patients group (>60 years old, with a mean θ-AATA of 58.6±5.6 degrees) and the younger patients group (≤60 years old, with a mean θ-AATA of 57.8±4.5 degrees) (P>0.05), but both of them are significantly larger than the generally deemed 45 degrees (P<0.01) (Figure 3C). Inter-observer and intra-observer variability in measuring the θ-AATA were 2.6 and 1.9 degrees respectively. The mean PLZ length measured at the θ-AATA (18.4±3.9) was substantially larger than that (15.9±3.1) obtained at the routine LAO-45 degrees (P<0.05).
Clinical results
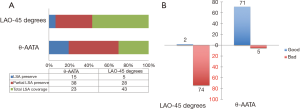
Technical success was achieved in all patients without any unexpected coverage of the supra-aortic vessels. If the PLZ was measured at routine LAO-45 degrees’ angle, according to the minimum 15-mm PLZ requirement, 5 patients could preserve LSA, 28 patients need partially LSA coverage and 43 patients require complete LSA coverage; while if the PLZ was measured at θ-AATA, 15 patients could preserve LSA, 38 patients could partially preserve LSA and only 23 patients need LSA total coverage (P<0.01) (Figure 4A). Finally, in our total LSA coverage group with 23 patients, 6 patients were given LSA chimney procedure to preserve the ante-grade blood flow since the left vertebral artery is the dominant one. Alignment of the proximal marks was achieved in 72 patients (93.4%) at θ-AATA and only 2 patients (2.7%) at normal LAO-45 degrees’ angle (P<0.01) (Figure 4B). No major type I endoleak (high volume) was found in the immediate post-deployment angiography. Mild type I endoleak (low volume) was detected in five patients, moderate type I endoleak (medium volume) was found in two patients. Of these seven patients with mild to moderate endoleak, “following and observing” policy was applied. The mean follow-up time was 23.4±5.8 months (range, 16–32 months). At 6-month time point’s follow-up, two of these with moderate endoleak had turned into mild endoleak and one of the five-mild type endoleak disappeared. The false lumen’s diameter of these patients almost remains unchanged. At the most up to date follow-up time point, two of these mild endoleak (four patients) disappeared. Due to these relative benign characteristics and the refusal of the further treatment from the corresponding patients, no secondary intervention procedure was applied and close follow-up was continued.
Discussion
Since firstly described by Nienaber and Dake in 1999, TEVAR has now been evolved as the preferred treatment choice for type B AD (7,8). Precise determination of the PLZ is of great importance to make the appropriate treatment strategy in terms of the possibility of TEVAR, position of stent-graft deployment and devices’ choice (9). According to the individual physicians’ preference, the measurement of PLZ could be based either on CTA scan or angiography (10). Generally, a LAO-45 degrees’ angle was estimated as the common θ-AATA, while our data showed that the exact average value of θ-AATA is 58.3±5.2 degrees, which is apparently larger than the normally applied 45 degrees (5). In this study, we utilized the index of proximal marks’ alignment to evaluate the accuracy and efficacy of this angle measuring method. The basic theory is that if the projecting angle is exactly perpendicular to the aortic arch, the projection of the proximal marks, which is annularly distributed, should be in one line, otherwise these points will scatter in different locations. If these marks are not in one line, the angle of C arm could be adjusted back and forth until eventually alignment is achieved. In this study we specifically chose the Medtronic thoracic stent-graft. The reason is that among the most widely available endografts in our center, Medtronic device has the relatively clearer proximal “8”-figure marks, which is easier to observe and check for alignment. Adopting this method, most of the patients (93.4%, 72 patients) had achieved the complete proximal marks’ alignment. However, at the routine LAO-45 degrees’ angle, only 2.7% (two patients) could get proximal marks’ alignment. Three possible reasons may contribute to that why there are still five patients could not obtain alignment even at θ-AATA in this study. The first mechanism is that the aortic arch may be partially deformed or displaced by the deployment of a relatively stiff stent-graft (5). The exact thoracic θ-AATA may be slightly altered accordingly. The second reason is that the angle measurement is based on the static CT scan, which randomly acquires the images of the aorta at any time in the cardiac cycle. However, the aorta will distend periodically and the distension is asymmetric due to the pulsatility and spiraling of the blood flow (11). The third reason is that CT scan is usually done several days before TEVAR, while the lesion could progress to varying degree and the actual angle might change a little bit subsequently (12).
According to the mathematical theory of trigonometric functions, the measured length of the PLZ is equal to Cos Δθ × exact length. Here this “Δθ” designates the discrepancy between θ-AATA and usually applied angiography angle (LAO-45 degrees). The value of Cos Δθ is negatively correlated to the degree of Δθ. When the actually applied angiography, angle is as same as the real θ-AATA, this Δθ is equal to zero, while the value of Cos Δθ is “1”. Under this angle, the PLZ could obtain its maximal projecting length. In our study, the measured PLZ had increased from 15.5±3.1 mm at the routine LAO-45 degrees’ angle angiography to 18.4±3.9 mm at θ-AATA. In terms of clinical strategy planning, if the routine LAO-45 degrees’ angiography had been applied, up to 43 patients need complete LSA coverage. Through adopting this new measuring method, only 23 patients need complete LSA coverage, while 53 patients could obtain total or partial LSA preserving.
LSA is one of the two vessels to supply the posterior cerebral circulation and the main supplying vessel of the left upper limb. If the left vertebral artery is the dominant one and the LSA has to be sacrificed, adjunctive procedures including chimney and bypass procedures are mandatory to reestablish its flow (4). Actually, in our series chimney technique was applied in six LSA-coverage-patients. Even though the current short to midterm studies did not reveal apparent severe detrimental effect to the cerebellum and left arm after the coverage of LSA under the condition that the left vertebral artery is not the dominant one (13-15). Some literature pointed out that direct coverage of LSA would increase the rate of stroke and the long-term insufficiency of the blood flow to the posterior circulation would eventually arouse accumulating side effect to the function of cerebellum (16,17). The newest meta-analysis on the stroke rate and LSA coverage during TEVAR revealed that the direct coverage group has a high rate of stroke than that of the more distal stent-graft placement group (7.4% vs. 4.0%, P<0.01) (18). Thereafter, try to preserve any LSA will be beneficial to the patients in the long-term viewpoint (19). In our series, adopting this new measuring method could improve the percentage of LSA preserving from 6.5% to 19.7%, the percentage of LSA partial preserving from 36.8% to 50%, while decrease the total LSA coverage ratio from 56.5% to 30.2%. The alteration of treatment strategy indicates that our new measuring method could potentially improve the rate of LSA preserving and benefit to achieve better clinical result consequently.
There are some specific software and other modalities could also be used to measure this θ-AATA. In terms of image analysis software, the most well-known one is Tera-Recon system (Aquarius, Foster City, CA, USA), which could efficiently manipulate DICOM data and provide an automated, user defined centerline inside the aorta and along with multi-planar axial reconstructions (MPR) orthogonal to this centerline path (20). This software also allows to rotate and examine the aorta in an unlimited number of projections. Together with the building-in sizing and measuring function, physicians could acquire any parameters including θ-AATA and curvature of the aortic arch. The other two softwares have the similar function are Simbionix PRORS (Simbionix USA Corp., Cleveland, OH, USA) and 3mensio (Pie Medical Imaging BV, Maastricht, The Netherlands) (21). However, some aspects may restrict them widely applying in developing countries. Firstly, they are highly charged for annual using and upgrading. Secondly, advanced hardware is needed to run them smoothly. Also, special training is required for familiar with manipulating. While our method is apparently cheaper, easier and more readily to use, especially under emergent situation.
Aiming to better understand the anatomical characteristic of the aortic arch, lots of studies on the analysis of aortic arch anatomical parameters had been carried out recently, with the study population consisted of patients either with aortic pathology or health controls (22-28). The most widely applied parameters included the diameter of different aorta segment, the clockwise angle and take off angle of the supra aortic branches, the type of aortic arch and the angle of the aortic curvature and etc. To the best knowledge of the authors, our paper is the first report to study θ-AATA and its potential influence on the clinical treatment planning and perioperative results.
This is a prospectively designed clinical study with a relative large sample size. There are some limitations of this study. First, this is a single center study. Multicenter study would be theoretically more convincing due to better bias elimination. Second, for better observation and confirmation of proximal marks’ alignment, Medtronic’s thoracic device is specifically selected. In further study, we will enroll the patients implanted with other endografts as well. Third, all the pre-operative CT scans are static. This may bring some bias to the measurement since the aorta will pulsatile and deform during cardiac cycle. Acquiring the images from the equal cardiac phase may potentially improve the measuring accuracy.
Conclusions
This study demonstrated that the exact θ-AATA is significantly larger than the generally deemed LAO-45 degrees. It is effective yet convenient to apply this CT based individual measuring method to measure the actual θ-AATA. Adopting this method could ensure to obtain the maximal length of PLZ, which is beneficial for optimal clinical treatment decision make and subsequently achieve better clinical results.
Acknowledgements
The authors would like to thank Ningyi Shao (Institute of Cardiovascular, Stanford University School of Medicine) for the support of statistical analysis; Mingxia Guo (Institute of Pediatric Cardiovascular, Stanford University School of Medicine) for the revision of grammar error; Yilan Sha (Hangzhou Institute of Landscape Architecture) for the drawing of the schematic diagram.
Funding: This work was supported by the National Natural Science Foundation of China (81570438) and the Young Scholar’s funding from the Health and Family Planning Committee of Shanghai (XYQ2013116).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of Zhongshan Hospital (No. 2017-204) and written informed consent was obtained from all patients.
References
- Pape LA, Awais M, Woznicki EM, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- Ehrlich MP, Rousseau H, Heijmen R, et al. Midterm results after endovascular treatment of acute, complicated type B aortic dissection: the Talent Thoracic Registry. J Thorac Cardiovasc Surg 2013;145:159-65. [Crossref] [PubMed]
- Caronno R, Piffaretti G, Tozzi M, et al. Intentional coverage of the left subclavian artery during endovascular stent graft repair for thoracic aortic disease. Surg Endosc 2006;20:915-8. [Crossref] [PubMed]
- Peterson BG, Eskandari MK, Gleason TG, et al. Utility of left subclavian artery revascularization in association with endoluminal repair of acute and chronic thoracic aortic pathology. J Vasc Surg 2006;43:433-9. [Crossref] [PubMed]
- Qu L, Raithel D. Techniques for precise thoracic endograft placement. J Vasc Surg 2009;49:1069-72. [Crossref] [PubMed]
- Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet 2015;385:800-11. [Crossref] [PubMed]
- Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med 1999;340:1539-45. [Crossref] [PubMed]
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999;340:1546-52. [Crossref] [PubMed]
- Alberta HB, Takayama T, Smits TC, et al. Aortic Arch Morphology and Aortic Length in Patients with Dissection, Traumatic, and Aneurysmal Disease. Eur J Vasc Endovasc Surg 2015;50:754-60. [Crossref] [PubMed]
- Goldstein SA, Evangelista A, Abbara S, et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2015;28:119-82. [Crossref] [PubMed]
- van Prehn J, Bartels LW, Mestres G, et al. Dynamic aortic changes in patients with thoracic aortic aneurysms evaluated with electrocardiography-triggered computed tomographic angiography before and after thoracic endovascular aneurysm repair: preliminary results. Ann Vasc Surg 2009;23:291-7. [Crossref] [PubMed]
- Baliga RR, Nienaber CA, Bossone E, et al. The role of imaging in aortic dissection and related syndromes. JACC Cardiovascular imaging 2014;7:406-24. [Crossref] [PubMed]
- McBride CL, Dubose JJ, Miller CC 3rd, et al. Intentional left subclavian artery coverage during thoracic endovascular aortic repair for traumatic aortic injury. J Vasc Surg 2015;61:73-9. [Crossref] [PubMed]
- Si Y, Fu W, Liu Z, et al. Coverage of the left subclavian artery without revascularization during thoracic endovascular repair is feasible: a prospective study. Ann Vasc Surg 2014;28:850-9. [Crossref] [PubMed]
- Klocker J, Koell A, Erlmeier M, et al. Ischemia and functional status of the left arm and quality of life after left subclavian artery coverage during stent grafting of thoracic aortic diseases. J Vasc Surg 2014;60:64-9. [Crossref] [PubMed]
- Zamor KC, Eskandari MK, Rodriguez HE, et al. Outcomes of Thoracic Endovascular Aortic Repair and Subclavian Revascularization Techniques. J Am Coll Surg 2015;221:93-100. [Crossref] [PubMed]
- Rizvi AZ, Murad MH, Fairman RM, et al. The effect of left subclavian artery coverage on morbidity and mortality in patients undergoing endovascular thoracic aortic interventions: a systematic review and meta-analysis. J Vasc Surg 2009;50:1159-69. [Crossref] [PubMed]
- Waterford SD, Chou D, Bombien R, et al. Left Subclavian Arterial Coverage and Stroke During Thoracic Aortic Endografting: A Systematic Review. Ann Thorac Surg 2016;101:381-9. [Crossref] [PubMed]
- Matsumura JS, Lee WA, Mitchell RS, et al. The Society for Vascular Surgery Practice Guidelines: management of the left subclavian artery with thoracic endovascular aortic repair. J Vasc Surg 2009;50:1155-8. [Crossref] [PubMed]
- Lee WA. Endovascular abdominal aortic aneurysm sizing and case planning using the TeraRecon Aquarius workstation. Vasc Endovascular Surg 2007;41:61-7. [Crossref] [PubMed]
- Velu JF, Groot Jebbink E, de Vries JP, et al. Validation of the Simbionix PROcedure Rehearsal Studio sizing module: A comparison of software for endovascular aneurysm repair sizing and planning. Vascular 2017;25:80-5. [Crossref] [PubMed]
- Alberta HB, Takayama T, Smits TC, et al. Aortic Arch Morphology and Aortic Length in Patients with Dissection, Traumatic, and Aneurysmal Disease. Eur J Vasc Endovasc Surg 2015;50:754-60. [Crossref] [PubMed]
- Chiu P, Lee HP, Venkatesh SK, et al. Anatomical characteristics of the thoracic aortic arch in an Asian population. Asian Cardiovasc Thorac Ann 2013;21:151-9. [Crossref] [PubMed]
- Pearce WH, Slaughter MS, LeMaire S, et al. Aortic diameter as a function of age, gender, and body surface area. Surgery 1993;114:691-7. [PubMed]
- Shin IY, Chung YG, Shin WH, et al. A morphometric study on cadaveric aortic arch and its major branches in 25 korean adults: the perspective of endovascular surgery. J Korean Neurosurg Soc 2008;44:78-83. [Crossref] [PubMed]
- Alberta HB, Secor JL, Smits TC, et al. Differences in aortic arch radius of curvature, neck size, and taper in patients with traumatic and aortic disease. J Surg Res 2013;184:613-8. [Crossref] [PubMed]
- Finlay A, Johnson M, Forbes TL. Surgically relevant aortic arch mapping using computed tomography. Ann Vasc Surg 2012;26:483-90. [Crossref] [PubMed]
- Malkawi AH, Hinchliffe RJ, Yates M, et al. Morphology of aortic arch pathology: implications for endovascular repair. J Endovasc Ther 2010;17:474-9. [Crossref] [PubMed]


