Advances in the use of motion management and image guidance in radiation therapy treatment for lung cancer
Introduction
Lung cancer is the leading cause of cancer-related deaths in the United States, the majority of which are from non-small cell lung cancer (NSCLC) (1). Radiation therapy (RT) is standard of care as a part of a multi-modality approach to treat locally advanced NSCLC (LA-NSCLC) and is being increasingly used in early stage NSCLC (ES-NSCLC) with stereotactic body radiation therapy (SBRT).
The goal for RT is to deliver sufficient dose to the tumor to provide control while minimizing the dose to highly sensitive surrounding organs at risks (OARs) in the thorax (lungs, heart, esophagus, spinal cord). Notable recent advances in RT treatment delivery recently have led to increasing importance of accurate tumor delineation, targeting and delivery.
The recent publication of results from the Radiation Therapy Oncology Group (RTOG) 0617 study has tempered excitement for dose escalation even with conformal techniques in locally advanced disease. This trial demonstrated worse survival rates with dose escalation and despite a dose of 74 Gy (compared with 60 Gy), 45.7% of patients failed locally and 38.4% failed regionally (2). Importantly, a robust multivariate analysis from this trial indicated that radiation dose to the heart and esophagitis/dysphagia were two factors most highly correlated with overall survival, highlighting the importance of minimizing dose to critical thoracic OARs. Subsequent institutional and retrospective efforts have particularly confirmed the link between heart exposure to radiation and survival, evincing a much higher rate of therapy-induced toxicities that previously thought (3,4). A secondary analysis of the RTOG 0617 study, demonstrated that IMRT was associated with lower rates of severe pneumonitis and cardiac doses, thereby justifying use of IMRT for LA-NSCLC, which improves target coverage while minimizing radiation to surrounding tissues (5). However, two unique challenges exist in the treatment of thoracic malignancies which include intra-fractional breathing motion and the potential for inter-fractional tumor changes and anatomical shifts.
Respiratory tumor motion increases the position uncertainty of the target and normal tissues in NSCLC, and achieving local tumor control requires understanding and incorporating tumor motion into the simulation, planning and delivery of RT, leading to multiple opportunities to monitor and mitigate motion during simulation and treatment. In addition, improving image guidance at the time of treatment offers the ability to increase effective tumor targeting thereby allowing a reduction in the planning target volume (PTV) margins resulting in decreased dose to OARs. Image-guided radiation therapy (IGRT) is broadly defined as the use of imaging during or just prior to radiation that verifies agreement of anatomy between the treatment plan and the patient, which when evaluated can verify or improve the accuracy of RT. Further, changes in anatomy during the course of radiation are common, particularly for thoracic malignancies, and alterations can lead to under-coverage of the intended target and/or overtreatment of OARs in both early-stage (6-8) and locally advanced NSCLC (9,10). With increasing conformity and understanding of anatomical changes during RT for lung cancer there has been increasing evaluation and incorporation of IGRT.
Appropriate delivery of RT for NSCLC is challenging, and many factors impact the effectiveness of treatment delivery including insufficient dose due to OAR constraints, inadequate target coverage from suboptimal alignment, and respiratory motion leading to intra-fractional and inter-fractional errors (11). Robust motion management and IGRT strategies are essential to optimize increasingly conformal treatments.
Motion management strategies
Introduction
Lung tumor and intra-thoracic normal tissue motion can have a significant impact on RT planning and delivery (12). While there are numerous uncertainties involved in accurately defining, targeting, computing, aligning, and finally delivering a prescribed radiotherapy treatment, methodologies have been developed to accommodate the ambiguity at each step of the process. For example, uncertainties in target delineation and the extent of microscopic (radiographically indistinct) disease are accounted for by expanding the treatment volume from the gross tumor volume (GTV) to the clinical target volume (CTV) (12-15). Due to the periodic respiratory cycle, one additional source of uncertainty in targeting and treating lung tumors is intra-fraction tumor motion. The internal target volume (ITV) is created to account for this uncertainty. Finally, The PTV margin accounts for patient setup errors and is designed to assure optimal target coverage.
There are five primary overarching class solutions to manage this problem: motion encompassment, respiratory gating, breath-hold, motion mitigation, and tumor tracking. These motion management and mitigation strategies have all been developed with three requirements: (I) clinical tolerability, (II) reduced probability of geometric miss, and (III) minimization of normal tissue exposure. It is also important to note that these techniques are not all mutually exclusive, and it is sometimes possible to combine multiple techniques.
Motion encompassment
The first class of solutions deals primarily with managing the uncertainty of respiratory motion by encompassing the entire range of tumor motion in the treated volume which mitigates the risk of missing the target while offering a solution that is straightforward to implement clinically. At its core, this technique involves identifying the complete extent of tumor motion and subsequently including all potential tumor locations as part of the treatment target. There are three potential ways to accomplish this, including slow CT, breath-hold CT and four-dimensional CT scanning (4DCT). Slow CT scans are obtained by performing CT scans across multiple cycles of respiration, and breath hold CT scans are obtained by performing CT scans at the two discrete points in the respiratory cycle, the end of expiration and the end of inspiration (16-18).
Both slow CT and breath-hold CT have largely been supplanted in clinics by the 4DCT (19,20). 4DCT scans allow the reconstruction of data into multiple discrete 3DCT scans that not only demonstrate the range of tumor motion over the normal breathing cycle, but also include data regarding the path of the tumor during the intervening phases of respiration. Furthermore, they are not as susceptible to the blurring of tissue margins that can render tumor boarders indistinct in the slow CT method. Additionally, unlike the other two techniques, the mode of acquisition of 4DCTs also leads to inherently collecting data regarding the relative time spent in the various phases of respiration, which is not uniformly distributed. Some drawbacks of 4DCT as compared to the other technique include additional infrastructure to obtain a 4DCT, patient and personnel training and also the increased workload in contouring, if performed on all the CT datasets. Alternate options of target delineation on average images, maximum intensity projection (MIP) images, or in cine-mode could conversely negate the benefit (21-23).
There is also persistent debate in literature regarding optimal reconstruction, particularly with regards to phase vs. amplitude binning (24). Additionally, some drawbacks of all these techniques include the fact that there is no way to accurately account for changes in breathing patterns between the time of image acquisition and treatment (25). Lastly, while each of these methods successfully accomplishes the first two goals of motion management (tolerable and reduction in geometric miss), these techniques in select cases could lead to increasing target volumes and, therefore, increased exposure of normal tissues.
Respiratory gating
Another option for managing respiratory tumor motion relies on creating a trigger to activate and deactivate treatment delivery or image acquisition, based either directly or indirectly on tumor position. This can broadly be conceptualized as: when the tumor enters a predefined region (controlled by operator-defined input parameters) the “gate” is open and X-rays are turned on; and when the tumor leaves that region the “gate” is closed and the X-rays are turned off.
While initially conceptualized in Japan in the 1980s (26,27), this technique has been studied across multiple centers and multiple clinical situations (28-30). In lung tumors, since the motion of the tumor is predominantly driven by respiratory cycles, numerous systems for approximating tumor position based on external surrogates for the respiratory cycle have been studied. These systems utilize external surrogates such as chest wall motion captured through the placement of external fiducial markers tracked by a couch or roof mounted camera system (31-33). More recently, products directly tracking the chest wall through optical surface monitoring also have been used (34). Lastly, a spirometer trace can be used to monitor and gate the beam on the basis of the volumetric measurement of air as the patient inhales and exhales (35). Alternatively, the actual tumor location either through the placement of internal fiducials or special rapid sequence imaging may offer options for gating directly based on tumor position (36-38).
There are a few concerns with use of respiratory gating that have limited its widespread adoption. First, respiratory-gating prolongs delivery time which can affect its clinical tolerability for patients and efficiency for treatment centers. Prolongation is dependent upon how narrow the trigger window is defined and can lead to significant durations of beam off time during each respiratory cycle. Treatment time is, therefore, in direct competition with precision of tumor position, and a balance must be obtained between faster treatments with larger treatment windows and more precise but longer treatments. Furthermore, unless gated at a single point, there is the potential for residual tumor motion within the temporal “gate” that must be accounted for by another technique (often motion-encompassing strategies). Lastly, even more so than motion-encompassing strategies, this technique can rely heavily on consistency of respiratory motion over time as the relationship between tumor position and chest wall/fiducial position may drift, leading to systematic uncertainty in the trigger signal and thereby potentially exacerbate the risk of geometric miss (39).
Breath-hold
Breath-hold during simulation and treatment is another option for respiratory management and requires active patient participation and trained therapists to coach and advise the patients during treatment. For breath-hold, the stability of tumor position and reproducibility of patient setup during each breath-hold need to be accurate and verified. Commonly used breath-hold methods are deep-inspiration breath hold (DIBH), mid-inspiration breath hold (MIBH), and active-breathing control (ABC). The former two self-held breath hold methods could be performed with or without respiratory monitoring.
In the DIBH and MIBH technique, it is preferred that the patient breathes through a spirometer with a nose clip to prevent nasal leakage. After determination of the inspiration capacity, the RT team will select a proportion of total lung capacity as a threshold level to ensure accurate positioning of the tumor. The patient undergoes a simulation CT scan under DIBH conditions, preferably monitored by a spirometer. During the simulation and treatment, the patient is instructed by visual coaching using video glasses to hold his/her breath to the specified threshold level. During treatment, the beam is turned on by the therapist when the target breath-hold level has been achieved and it is turned off immediately by the therapist, or automatically using a gating module, with excessive deviation.
Another breath-hold method is the ABC system that is commonly used in treating left-sided breast cancer to protect the heart without compromising coverage of the target (40). Similar to DIBH, a spirometer is used and is connected to a valve measuring the respiratory level. Prism glasses display the ABC screen to the patient. Prior to simulation the patient takes a few deep breaths which provide the technical setting measurements needed as well as the breath-hold level. During simulation and treatment, as the patient holds his/her breath at the specific level the valve is closed to block the patient’s breath. Considering patient comfort during the course of treatment, it has been shown that the stable and reproducible tumor position can be achieved at 75% of the deep inspiratory level (41).
The self-held breath-hold without respiratory monitoring has also been used in clinic for broader target volumes like breast/chest wall irradiation. For deeper tumors, a 4DCT scan can be done to characterize the tumor motion during the breath hold. During treatment a communication button (42,43) is given to the patient which can be used to clear beam interlock and indicate he/she is at breath-hold level and alert the therapists to turn on the radiation beam. This method can also be performed with respiratory monitoring systems and turn off the beam if the patient exits the breath-level.
Motion mitigation
NICAIAlong the same line as breath-hold, there are a set of techniques that can be grouped into the category of motion mitigation. Unlike breath-hold techniques, these methods allow the patient to continue breathing throughout the course of treatment. Through various methodologies, however, the patient’s breathing is rendered shallower in an effort to reduce the effects on tumor motion. These techniques may also have the additional benefit of making the tumor motion more regular or reduce imaging artifacts for patients with long expiratory phases/slow breathing.
One of the primary ways to accomplish motion mitigation is through forced shallow breathing achieved most commonly through abdominal compression. This technique has been used in the treatment of both lung and intra-abdominal tumors, particularly in the setting of SBRT (44,45). Abdominal compression can be performed using a stereotactic body frame or a belt-like device. Both devices exert a controlled and constant pressure on the patients’ abdomen, thereby reducing diaphragmatic motion, which is the predominant driver of the superior-inferior component of respiratory tumor motion. Patient selection is important in isolating the benefit of these techniques, which are predominantly useful in patients with pre-mitigation tumor excursions of >8–10 mm. This technique can be combined with other techniques such as placement of fiducials or 4DCT with and without forced-shallow breathing devices to help determine if there is successful motion mitigation. Furthermore, other motion encompassing techniques may still be necessary to account for the residual motion in treatment planning.
Tumor tracking
There are multiple technological systems developed to localize and monitor the target in real time. The first one is direct tumor imaging by means of radiographic or fluoroscopic images. Due to low contrast of tumor versus surrounding tissue, this method may work only for some lung tumors. Multiple fiducial markers with high atomic number (typically gold) can be used to improve the contrast for tumor localization and image registration in treating liver, pancreas, prostate, and lung tumors (46).
Another method for tumor tracking is based on breathing signals or body surface motion. This can be done using the optical surface tracking system or infrared (IR) tracking system. The optical tracking technology typically consists of two or more camera pods that project and detect the reflection of a pattern on the patient’s body providing a real-time 3D surface imaging. This system has been evaluated for tumor tracking in thoracic and abdominal tumor sites (47). The IR tracking system is similar to a 4DCT system in conjunction with X-ray imaging systems and can be used for accurate patient positioning as well as real-time patient monitoring during treatment (48). It provides a signal to move the couch and hold the beam if the patient moves during the respiratory gating procedure. Multiple studies have investigated tumor margin reduction and the accuracy of monitoring lung tumor motion using an IR tracking system (49-52). The main disadvantage of both IR and optical surface tracking systems is that the systems provide external patient motion that is not a perfect representation of internal organ/target motion.
The other non-radiographic system uses electromagnetic technology to track radiofrequency (RF) of transponders implanted in the target by means of a 4D electromagnetic array. Briefly, the transponders receive and re-emit the RF signal emitted from an electromagnetic array. This signal is detected and allows the system to locate transponders relative to the electromagnetic array. The main disadvantage of RF tracking systems is the accuracy of target localization in the presence of surrounding magnetic fields or any metal objects. The application of RF tracking systems has been investigated for the treatment of prostate and lung cancers (53-59).
Image guidance
Introduction
A complementary approach to motion management for improving treatment accuracy and decreasing OAR dose is the use of IGRT at the time of treatment. LA-NSCLC has historically required large PTV margins due to frequent alignment issues and inter-fractional anatomical changes in an effort to ensure adequate target dose coverage (Figure 1). However, increasing PTV margins can lead to increased irradiation of normal surrounding structures and may limit the ability to safely dose escalate. The use of IGRT has two-fold objectives depending on the image-guidance used. Accurate alignment can be achieved with on-board orthogonal kilovolt (kV)/megavolt (MV) X-rays or cone-beam (CB) kV/MV CT scans. CBCT adds the ability to identify anatomical changes such as tumor response or normal tissue changes (effusion, collapse, shifts). Both of these can lead to more accurate treatment delivery with the possibility to decrease PTV margins.
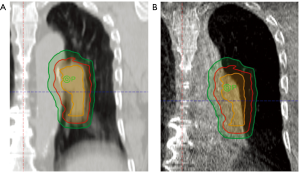
Errors accounted for in the PTV include systemic errors that affect all treatments (e.g., laser alignment, organ position on imaging, target identification error) and random errors that affect individual treatments (e.g., set-up error and organ motion). Margin calculation formulas have been proposed and evaluated which utilize formulas weighting systemic and random errors to determine expansions (60). Typical recommendations for a setup margin from consensus guidelines of 5 mm, for example, can almost double the volume of irradiated tissue. In this section, we discuss methods used to image patients during treatment with the goal of decreasing setup errors and subsequently decreasing dose to OARs and increasing certainty in tumor targeting.
Imaging modalities
Early image guidance relied on planar imaging with film and then electronic portal imaging devices (EPIDs). MV imaging allows for verification of the treatment field but has limited image quality and relatively high imaging dose. Use of kV X-ray sources and EPIDs offset at right angles to the gantry have been widely adopted due to their superior image quality and lower dose. In addition to their use for pre-treatment static imaging, mounted orthogonal EPIDs are also used as a tracking device to track high density materials including bone and fiducials (37,38,61), independent of beam-on time. The CyberKnife system (Accuray, Sunnyvale, CA, USA) includes room-mounted orthogonal EPIDs and treatment with a 6-MV linear accelerator (LINAC) on a robotic arm, allowing for movement of the LINAC with tumor motion in real time (61). Limitations include imaging dose, fiducial requirement, lack of volumetric-based imaging, and directional beam entrance limitations.
Initial use of volumetric based imaging was performed with on-board CT-on-rails systems in which the patient first underwent imaging in treatment position and then transported directly to treatment unit on the same couch. Although this system was feasible and allowed for corrections based on comparison with the treatment CT scan, CT on rails has been largely supplanted by linear accelerators modified or developed with onboard volumetric cone beam CT (CBCT) techniques that use KV imaging or tomotherapy units which generate MV CT images. CBCT (kV or MV) acquires planar images while rotating around the patient which are reconstructed into volumetric images (62). While image quality is reduced compared to diagnostic CTs, the patient remains in place throughout imaging, registration, verification and treatment, allowing for identification and correction of positional errors (shift, rotation, and deformation) (63). CBCT use allows for patient setup based on evaluation of the 3D soft tissue anatomy in the region of interest (64). CBCT with kV provides images with sharper contrast but is affected more by metal artifacts, compared to MV CBCTs (65).
CBCTs have been shown to significantly improve accuracy of targeting and reduction in errors (66-68). Limitations to CBCT include helical scattering and decrease imaging quality. Also, because the gantry rotates slowly, respiratory motion artifacts are included in the resultant image. Respiratory correlated 4D CBCT has been developed as a method to overcome this limitation and uses the relative position of the diaphragm to bin respiratory phases during reconstruction (69). Use of 4D CBCT demonstrated that the change in amplitude of motion day to day was 1 mm or less on average in all directions (7,69,70). In another study evaluating the use of 4DCT, 4D-IGRT and beam gating in lung cancer, the margin reduction for 4DCT was 0–38% while the addition of 4D-IGRT reduced margins 37–47% with little additional benefit for beam gating (71).
Adaptive radiotherapy
Changes in target location, size or density observed during imaging guidance can lead to alterations in the dose distribution within the PTV and/or OARs; therefore, adaptation of the radiation treatment plan may be utilized for improved treatments in real time. While image guidance affords the opportunity to monitor the coverage of the target with respect to the treatment volume, adaptive radiotherapy attempts to incorporate observed changes in the target volume into the treatment which theoretically allows for decreased margins over the entirety of a treatment for LA-NSCLC. Tumors may cause or lead to bronchial obstruction, atelectasis, pneumonia, or pleural effusions, leading to anatomical shifts of the target and/or OARs during an RT course. Volumetric imaging can identify these changes and allow for alteration in alignment, evaluation of target coverage and signal consideration for re-planning. Careful selection of structures for alignment may allow for improved target coverage in the case of anatomical changes and alterations in soft tissues that may not be reflected in bony anatomy.
While adapting for tumor shrinkage allows for decreasing margins during treatment, the question of whether this allows for adequate dose to microscopic tumor extension remains largely unanswered. Still, multiple groups have reported on implementation of adaptive radiotherapy paradigms in the treatment of LA-NCSLC. Knap et al. reported that one third of patients undergoing treatment achieved a significant tumor shrinkage at the conclusion of radiotherapy (72). Kwint et al. evaluated 177 patients undergoing definitive radiotherapy, and evaluation of CBCTs demonstrated that 72% of patients had intra-thoracic anatomical changes (73). Similarly, they report that 36% percent of patients had significant tumor shrinkage. Twelve percent of the total 210 changes were deemed to require plan alterations prior to treatment for the same day. Møller et al. report on a prospective incorporation of adaptive radiotherapy into the treatment of 233 patients in which anatomical changes prompted adaptation of the plan (74). They reported that 63 of 233 (27%) of patients underwent re-planning, of which 75% were adjusted for a decrease in tumor dose.
IGRT in NSCLC—practical issues
Image registration of the treatment planning scan and the pre-treatment image guidance allows for the ability to make corrections based on target or OAR positioning. However, there may be inherent risks of matching to one or the other. While matching to the vertebral bodies minimizes risk of inadvertent dose changes to the spinal cord, it has also been shown to be less effective compared to soft tissue alignment for target coverage. Shifts between fractions of the primary tumor to vertebral body can vary by as much as 5–7 mm but have been shown to be as high as 3 cm (6,75). Lavoie et al. evaluated target coverage during the course of RT using tattoo alignment as well as IGRT aligned to the vertebral bodies and carina. While their work suggested improvement by both IGRT alignments over tattoos, carina alignment had improved target coverage compared to bony alignment (76). A report from the Princess Margaret Hospital evaluated alignment to the vertebral bodies versus the carina and demonstrated that alignment to the carina allows for a quick, reproducible and improved target coverage (64). Conversely, response during treatment may lead to resolution of atelectasis with equal risk of tumor under-coverage, suggesting a need to modify treatment for this scenario as well, which can occur in 26% of patients (77).
Frequency of image guidance has also been evaluated. For example, a comparison between daily IGRT using CBCT and less frequent imaging was performed by the Princess Margaret Hospital with determination of PTV margins required for sufficient coverage. When image guidance was used daily, PTV margins of 3–4 mm were sufficient, compared to less than daily imaging, which required PTV margins ranging from 5–9 mm (78). Yeung et al. evaluated CBCT on multiple frequencies and demonstrated PTV margins ranging from 1–1.6 cm for no image guidance to 4–5 mm with CBCT every other day (79).
SBRT for early-stage NSCLC involves delivering very high doses of RT per fraction to a target of known 3D coordinates and poses unique IGRT challenges. The proximity of lung tumors to nearby normal structures like the heart, esophagus, and spinal cord requires precision when delivering large fraction sizes. Moreover, smaller tumors especially in lower lobe are more likely to be mobile compared to locally advanced tumors, exacerbating respiratory motion changes. Evidence suggests that the use of stereotactic body frames are not as accurate as image guidance and consequently, motion management and image guidance are essential for the safe delivery of SBRT (6,7). While matching bony landmarks by using X-ray based kV imaging is widely prevalent even for SBRT treatments in the thorax, studies have shown that matching soft tissue or tumor by using CBCT based imaging can be preferable. Prolonged delivery time during CBCT-based SBRT could potentially introduce concerns regarding intra-fraction motion of the mean target position. Bissonnette et al. studied the importance of CBCT in SBRT for lung tumors where initial set up accuracy using CBCT was followed by manual and remote controlled couch adjustments for positioning discrepancy more than 3 mm in any direction (66). They demonstrated that positioning errors more than 5 mm occur in 54% of all fractions and more than 10 mm in 25% of the all delivered fractions. Using CBCT, single positional correction helped to achieve accuracy in position in 82%, and a second correction was required in 18% of all the fractions to achieve a 3 mm tolerance. Corradetti et al. examined the accuracy of kV X-ray matching to bony anatomy compared to CBCT based tumor matching. They also studied the intra-fractional tumor motion by immediate post treatment CBCT. They report suboptimal coverage when matching to bony landmarks when a tumor margin of 3–5 mm is used. They also show that after a careful matching of tumor localization using CBCT, about 27% of the fractions required intra-fraction shifts of 3 mm or greater (80). Thus, while there are multiple ways to mitigate the intra-fractional motion, it is important to reduce the possible errors using CBCT.
Special considerations for protons
Proton therapy is rapidly expanding in availability worldwide. Uptake has especially accelerated with the advent of active scanning delivery techniques and integrated image guidance. Protons offer a unique dose distribution due to the inherent finite range of the beam and consequent improved sparing of critical structures with similar oncologic efficacy. In the thorax, a number of OARs are present—the uninvolved lung, heart, esophagus, major vessels, chest wall, spinal cord, etc. Each of these when exposed to high doses of radiotherapy, or even low doses in some cases, can be profoundly affected with ensuing substantial, clinically meaningful toxicities.
Proton therapy, and especially intensity-modulated proton therapy (IMPT) as delivered by pencil beam scanning (PBS) techniques, offers the promise of dose-escalation while often substantially decreasing the exposure to normal tissues. Numerous dosimetric planning studies have been previously reviewed demonstrating benefits in both early stage and locally advanced disease (81,82). Unfortunately, the only randomized data to date, in the form of a conglomerate phase III trial between MD Anderson Cancer Center and Massachusetts General Hospital that predated the use of PBS or CBCT-based image-guidance for proton therapy, demonstrated similar outcomes between IMRT and 3D-conformal proton therapy in both toxicity and disease control, although patients in the proton arm were noted to have larger target volumes and higher prescribed doses (83).
Further investigation is clearly warranted into the clinical benefits that proton therapy may offer in the treatment of lung cancer. However, there is little doubt that more rigorous image guidance and motion mitigation strategies are required for the accurate and effective delivery of proton therapy in the thorax.
Motion mitigation in proton therapy
Proton therapy is particularly sensitive to setup inaccuracies and density changes in the path of the beam. The finite range of the beam lends the superior dose-distributions achievable but also adds uncertainty in target coverage. Hence, it is even more critical when using proton therapy to properly map and understand the full path of the tumor target especially throughout the breathing cycle in the case of intrathoracic tumors. Four-dimensional simulation techniques for treatment planning can help estimate motion and should regularly be employed. However, with the shift towards PBS delivery techniques, the additional challenge of the interplay effect has arisen and dominated innovations for proton therapy in recent years.
Interplay effect describes the phenomenon that since the target is moving during the breathing cycle and the beam is being actively scanned across the treatment field, the two almost assuredly will not be sufficiently synchronized to give the projected dose distribution across the target, especially if there is significant motion in the target (>5 mm–1 cm) (84). As a result, a motion mitigation strategy (or several) is clearly needed when treating moving targets. There are several effective strategies for reducing the motion of the target during the breathing cycle as previously described, and reduced tumor motion has been linked with improved target coverage and dose distribution in dosimetric and in vitro simulations (85).
Several additional strategies can be employed in PBS proton therapy to mitigate the interplay effect (86). Rescanning divides each fraction of radiotherapy into sub-fractions that are sequentially delivered, thus separating the fraction of delivery over multiple breathing cycles, effecting a more homogeneous dose across the target (87). Rescanning can be achieved by volumetric (delivering to the entire target volume several times consecutively) or layered (delivering to each energy layer several times consecutively) approaches, each of which has strengths and weaknesses. Intentionally increasing the spot size, often by introducing a range-unnecessary range shifter, can also make a PBS plan substantially less sensitive to tumor motion but at the cost of loss of conformality and concern for increased dose to normal tissues caused by inter play effect (88). Spot spacing, gating, and breathing speed/pattern manipulation can also improve dose delivery and matching to the intended distribution.
If a moving target can be made relatively immobile, this would maximally reduce the interplay effect. Respiratory gating and breath-hold techniques are theoretically desirable but logistically challenging, especially in large centers with a single proton source/accelerator and multiple treatment rooms and in patients with poor lung function. While still being investigated, the use of high frequency jet ventilation, in which the patient’s breathing excursion is virtually stopped by the administration of rapid, low tidal volume gas exchanges during simulation and treatment, may be an intriguing way forward for the delivery of proton therapy (89).
Image guidance in proton therapy
In addition to effective motion management strategies, effective image guidance and regular anatomical assessment are also key when using proton beam therapy for thoracic cancers. Of particular concern in the thorax are changes in tumor, lung tissue, and pleural effusions. As an example, lung consolidation can change the location of a target, but with PBS proton treatment, the change in tissue density can dramatically alter dose delivery. In Figure 2, lung consolidation changed the target location; however tissue density changes also dramatically decreased superior posterior target coverage even in areas previously well covered (arrow). Proton therapy is often employed in clinical situations in which an OAR lies in close proximity to a target structure or when thoracic re-irradiation is employed, making assuredness in dose-delivery even more critical.
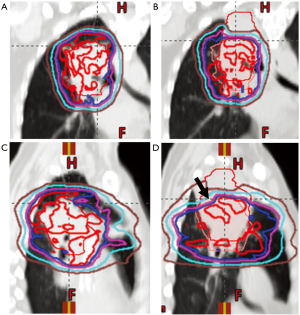
In the cases of tumor swelling/enlargement or pleural effusions arising during treatment, under-dosage at the distal portion of each beam’s range may be evident. If the tumor responds/shrinks or an effusion dissipates during therapy, over-ranging into critical structures can occur. As a result, volumetric imaging should be obtained regularly throughout the course of therapy to assess for changes in the target and surrounding structures. This can be achieved by on-board CBCT scanning when available (90) and/or by repeat CT scanning regularly throughout the course of therapy. When a verification or quality assurance CT (QACT) scan is obtained, this also gives the opportunity for fusion to the initial planning CT, reapplication of the dose, and assessment of perturbations of the dose distribution based on anatomical changes.
Tumors in the thorax and lungs are particularly challenging with regards to range certainty due to the significant heterogeneities in tissue density in the region and the significant changes that can occur in density based on tumor position, tumor motion, lung tissue changes, pleural effusions, among others (91,92). The failure rates of the Imaging and Radiation Oncology Core Houston’s (IROC) anthropomorphic phantom tests are a testament to these challenges (93). Proton therapy institutions most commonly struggled to pass the liver and lung phantom tests with only a 63% overall pass rate, substantially less than the 85% for the static phantoms of the head/neck, spine, and prostate. Noted in IROC’s report were the issues of multiple tumors, heterogeneities, and motion that led to most of the failures.
Although some allotment is made for inaccuracies in range calculation during proton therapy planning—usually in the form of a distal and proximal target margin encompassing a tissue range uncertainty of approximately 3.5%—anatomical changes in the beam path such as the appearance of a previously absent pleural effusion can vastly exceed this adjustment. Therefore, only regular volumetric imaging throughout the course can aid the clinical team in making the necessary adjustments should changes arise. If it is possible to foresee an area at risk for anatomical changes (such as the presence of a pleural effusion at the time of simulation that may wax/wane), beam paths through this area should be avoided when feasible. Additionally, integration of Monte Carlo based dose algorithms into the treatment planning software should further improve the accuracy of projected dose distributions over pencil beam algorithm techniques (94,95).
Recent developments in the field of in vivo range verification may further aid in proton therapy delivery and, in the near future, may provide substantially improved real-time information to clinicians as to whether treatment plan adjustments should be made based on anatomical changes. Currently, the most frequently employed technique utilizes positron emission tomography (PET) imaging to visualize the activation of the patient’s tissue by the proton beam (96,97). Prompt gamma imaging measures the prompt gamma radiation from proton beam nuclear excitation and offers improved spatial accuracy as to the position of the Bragg peak and dose falloff (98,99). However, clinically feasible detectors for this method remain a challenge. While these methods are not widely clinically available as yet, they could be an important tool in the near future for verification of proton beam range during a treatment course.
Conclusions
With growing evidence underscoring the importance of minimizing dose to OARs while performing thoracic RT, continued evaluation and incorporation of motion management strategies and image guidance hold promise for advancing treatment for NSCLC. However, the introduction of these technological advances must be performed in a manner that recognizes patient comfort and reproducibility, consistency in varying clinical environments and safeguards against missing targets with tighter margins. As the field continues to move towards increasingly conformal techniques, implementation becomes more important and opportunities will continue to arise to decrease dose to surrounding organs while potentially allowing for dose escalation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Wang K, Eblan MJ, Deal AM, et al. Cardiac toxicity after radiotherapy for stage III Non–Small-cell lung cancer: Pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol 2017;35:1387-94. [Crossref] [PubMed]
- Speirs CK, DeWees TA, Rehman S, et al. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non–small cell lung cancer. J Thorac Oncol 2017;12:293-301. [Crossref] [PubMed]
- Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non–small-cell lung cancer: A secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol 2017;35:56-62. [Crossref] [PubMed]
- Guckenberger M, Meyer J, Wilbert J, et al. Cone-beam CT based image-guidance for extracranial stereotactic radiotherapy of intrapulmonary tumors. Acta Oncol 2006;45:897-906. [Crossref] [PubMed]
- Worm ES, Hansen AT, Petersen JB, et al. Inter-and intrafractional localisation errors in cone-beam CT guided stereotactic radiation therapy of tumours in the liver and lung. Acta Oncol 2010;49:1177-83. [Crossref] [PubMed]
- Sonke JJ, Rossi M, Wolthaus J, et al. Frameless stereotactic body radiotherapy for lung cancer using four-dimensional cone beam CT guidance. Int J Radiat Oncol Biol Phys 2009;74:567-74. [Crossref] [PubMed]
- Schaake EE, Rossi MM, Buikhuisen WA, et al. Differential motion between mediastinal lymph nodes and primary tumor in radically irradiated lung cancer patients. Int J Radiat Oncol Biol Phys 2014;90:959-66. [Crossref] [PubMed]
- Hoffmann L, Holt MI, Knap MM, et al. Anatomical landmarks accurately determine interfractional lymph node shifts during radiotherapy of lung cancer patients. Radiother Oncol 2015;116:64-9. [Crossref] [PubMed]
- Cho BC, Bezjak A, Dawson LA. In: Image guidance in Non-Small cell lung cancer. Semin Radiat Oncol 2010;20:164-70. [Crossref] [PubMed]
- Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM task group 76. Med Phys 2006;33:3874-900. [Crossref] [PubMed]
- Van de Steene J, Linthout N, de Mey J, et al. Definition of gross tumor volume in lung cancer: Inter-observer variability. Radiother Oncol 2002;62:37-49. [Crossref] [PubMed]
- Giraud P, Elles S, Helfre S, et al. Conformal radiotherapy for lung cancer: Different delineation of the gross tumor volume (GTV) by radiologists and radiation oncologists. Radiother Oncol 2002;62:27-36. [Crossref] [PubMed]
- Bowden P, Fisher R, Mac Manus M, et al. Measurement of lung tumor volumes using three-dimensional computer planning software. Int J Radiat Oncol Biol Phys 2002;53:566-73. [Crossref] [PubMed]
- Lagerwaard FJ, Van Sornsen de Koste JR, Nijssen-Visser MR, et al. Multiple “slow” CT scans for incorporating lung tumor mobility in radiotheraphy planning. Int J Radiat Oncol Biol Phys 2001;51:932-7. [Crossref] [PubMed]
- de Koste JR, Lagerwaard FJ, de Boer HC, et al. Are multiple CT scans required for planning curative radiotherapy in lung tumors of the lower lobe? Int J Radiat Oncol Biol Phys 2003;55:1394-9. [Crossref] [PubMed]
- de Koste JR, Lagerwaard FJ, Schuchhard-Schipper RH, et al. Dosimetric consequences of tumor mobility in radiotherapy of stage I non-small cell lung cancer–an analysis of data generated using ‘slow’CT scans. Radiother Oncol 2001;61:93-9. [Crossref] [PubMed]
- Vedam SS, Keall P, Kini V, et al. Acquiring a four-dimensional computed tomography dataset using an external respiratory signal. Phys Med Biol 2003;48:45. [Crossref] [PubMed]
- Ford EC, Mageras G, Yorke E, et al. Respiration-correlated spiral CT: A method of measuring respiratory-induced anatomic motion for radiation treatment planning. Med Phys 2003;30:88-97. [Crossref] [PubMed]
- Rietzel E, Liu AK, Chen GT, et al. Maximum-intensity volumes for fast contouring of lung tumors including respiratory motion in 4DCT planning. Int J Radiat Oncol Biol Phys 2008;71:1245-52. [Crossref] [PubMed]
- Weiss E, Wijesooriya K, Ramakrishnan V, et al. Comparison of intensity-modulated radiotherapy planning based on manual and automatically generated contours using deformable image registration in four-dimensional computed tomography of lung cancer patients. Int J Radiat Oncol Biol Phys 2008;70:572-81. [Crossref] [PubMed]
- Park K, Huang L, Gagne H, et al. Do maximum intensity projection images truly capture tumor motion? Int J Radiat Oncol Biol Phys 2009;73:618-25. [Crossref] [PubMed]
- Becker N, Quirk S, Kay I, et al. A comparison of phase, amplitude, and velocity binning for cone-beam computed tomographic projection-based motion reconstruction. Pract Radiat Oncol 2013;3:e209-17. [Crossref] [PubMed]
- Redmond KJ, Song DY, Fox JL, et al. Respiratory motion changes of lung tumors over the course of radiation therapy based on respiration-correlated four-dimensional computed tomography scans. Int J Radiat Oncol Biol Phys 2009;75:1605-12. [Crossref] [PubMed]
- Ohara K, Okumura T, Akisada M, et al. Irradiation synchronized with respiration gate. Int J Radiat Oncol Biol Phys 1989;17:853-7. [Crossref] [PubMed]
- Tada T, Minakuchi K, Fujioka T, et al. Lung cancer: Intermittent irradiation synchronized with respiratory motion--results of a pilot study. Radiology 1998;207:779-83. [Crossref] [PubMed]
- Minohara S, Kanai T, Endo M, et al. Respiratory gated irradiation system for heavy-ion radiotherapy. Int J Radiat Oncol Biol Phys 2000;47:1097-103. [Crossref] [PubMed]
- Hara R, Itami J, Kondo T, et al. Stereotactic single high dose irradiation of lung tumors under respiratory gating. Radiother Oncol 2002;63:159-63. [Crossref] [PubMed]
- Kubo HD, Wang L. Compatibility of varian 2100C gated operations with enhanced dynamic wedge and IMRT dose delivery. Med Phys 2000;27:1732-8. [Crossref] [PubMed]
- Duan J, Shen S, Fiveash JB, et al. Dosimetric effect of respiration-gated beam on IMRT delivery. Med Phys 2003;30:2241-52. [Crossref] [PubMed]
- Ramsey CR, Cordrey IL, Oliver AL. A comparison of beam characteristics for gated and nongated clinical x-ray beams. Med Phys 1999;26:2086-91. [Crossref] [PubMed]
- Nakagawa K, Haga A, Kida S, et al. 4D registration and 4D verification of lung tumor position for stereotactic volumetric modulated arc therapy using respiratory-correlated cone-beam CT. J Radiat Res 2013;54:152-6. [Crossref] [PubMed]
- Li G, Wei J, Huang H, et al. Characterization of optical-surface-imaging-based spirometry for respiratory surrogating in radiotherapy. Med Phys 2016;43:1348-60. [Crossref] [PubMed]
- Hoisak JD, Sixel KE, Tirona R, et al. Correlation of lung tumor motion with external surrogate indicators of respiration. Int J Radiat Oncol Biol Phys 2004;60:1298-306. [Crossref] [PubMed]
- Shimizu S, Shirato H, Ogura S, et al. Detection of lung tumor movement in real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys 2001;51:304-10. [Crossref] [PubMed]
- Seppenwoolde Y, Shirato H, Kitamura K, et al. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys 2002;53:822-34. [Crossref] [PubMed]
- Shirato H, Shimizu S, Kitamura K, et al. Four-dimensional treatment planning and fluoroscopic real-time tumor tracking radiotherapy for moving tumor. Int J Radiat Oncol Biol Phys 2000;48:435-42. [Crossref] [PubMed]
- Malinowski K, McAvoy TJ, George R, et al. Incidence of changes in respiration-induced tumor motion and its relationship with respiratory surrogates during individual treatment fractions. Int J Radiat Oncol Biol Phys 2012;82:1665-73. [Crossref] [PubMed]
- Wong JW, Sharpe MB, Jaffray DA, et al. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys 1999;44:911-9. [Crossref] [PubMed]
- Remouchamps VM, Letts N, Vicini FA, et al. Initial clinical experience with moderate deep-inspiration breath hold using an active breathing control device in the treatment of patients with left-sided breast cancer using external beam radiation therapy. Int J Radiat Oncol Biol Phys 2003;56:704-15. [Crossref] [PubMed]
- Kitamura K, Shirato H, Onimaru R, et al. Feasibility study of hypofractionated gated irradiation using a real-time tumor-tracking radiation therapy system for malignant liver tumors. Int J Radiat Oncol Biol Phys 2002;54:125-6. [Crossref]
- Suramo I, Päivänsalo M, Myllylä V. Cranio-caudal movements of the liver, pancreas and kidneys in respiration. Acta Radiol Diagn (Stockh) 1984;25:129-31. [Crossref] [PubMed]
- Lax I, Blomgren H, Näslund I, et al. Stereotactic radiotherapy of malignancies in the abdomen: Methodological aspects. Acta Oncol 1994;33:677-83. [Crossref] [PubMed]
- Lin L, Souris K, Kang M, et al. Evaluation of motion mitigation using abdominal compression in the clinical implementation of pencil beam scanning proton therapy of liver tumors. Med Phys 2017;44:703-12. [Crossref] [PubMed]
- Shirato H, Harada T, Harabayashi T, et al. Feasibility of insertion/implantation of 2.0-mm-diameter gold internal fiducial markers for precise setup and real-time tumor tracking in radiotherapy. Int J Radiat Oncol Biol Phys 2003;56:240-7. [Crossref] [PubMed]
- Hughes S, McClelland J, Tarte S, et al. Assessment of two novel ventilatory surrogates for use in the delivery of gated/tracked radiotherapy for non-small cell lung cancer. Radiother Oncol 2009;91:336-41. [Crossref] [PubMed]
- Gupta T, Phurailatpam R, Ajay M, et al. Quality assurance and commissioning of an infrared marker-based patient positioning system for frameless extracranial stereotactic radiotherapy. Int J Biomed Sci 2007;3:298-301. [PubMed]
- Matney JE, Parker BC, Neck DW, et al. Target localization accuracy in a respiratory phantom using BrainLab ExacTrac and 4DCT imaging. J Appl Clin Med Phys 2011;12:3296-9. [Crossref] [PubMed]
- Korreman SS, Juhler-Nøttrup T, Boyer AL. Respiratory gated beam delivery cannot facilitate margin reduction, unless combined with respiratory correlated image guidance. Radiother Oncol 2008;86:61-8. [Crossref] [PubMed]
- Yorke E, Rosenzweig KE, Wagman R, et al. Interfractional anatomic variation in patients treated with respiration‐gated radiotherapy. J Appl Clin Med Phys 2005;6:19-32. [Crossref] [PubMed]
- Vedam SS, Kini V, Keall P, et al. Quantifying the predictability of diaphragm motion during respiration with a noninvasive external marker. Med Phys 2003;30:505-13. [Crossref] [PubMed]
- Balter JM, Wright JN, Newell LJ, et al. Accuracy of a wireless localization system for radiotherapy. Int J Radiat Oncol Biol Phys 2005;61:933-7. [Crossref] [PubMed]
- Kupelian P, Willoughby T, Litzenberg D, et al. Clinical experience with the calypso® 4D localization system in prostate cancer patients: Implantation, tolerance, migration, localization and real time tracking. Int J Radiat Oncol Biol Phys 2005;63:S197. [Crossref]
- Litzenberg DW, Willoughby TR, Balter JM, et al. Positional stability of electromagnetic transponders used for prostate localization and continuous, real-time tracking. Int J Radiat Oncol Biol Phys 2007;68:1199-206. [Crossref] [PubMed]
- Mayse M, Smith R, Park M, et al. Development of a non-migrating electromagnetic transponder system for lung tumor tracking. Int J Radiat Oncol Biol Phys 2008;72:S430. [Crossref]
- Mayse M, Peauroi J, Parikh P, et al. Long-term interaction and tissue response of a bronchoscopically implanted, anchored electromagnetic transponder in the canine lung. Int J Radiat Oncol Biol Phys 2009;75:S37. [Crossref]
- Murphy MJ, Eidens R, Vertatschitsch E, et al. The effect of transponder motion on the accuracy of the calypso electromagnetic localization system. Int J Radiat Oncol Biol Phys 2008;72:295-9. [Crossref] [PubMed]
- Parikh P, Hubenschmidt J, Dimmer S, et al. 4D verification of real-time accuracy of the calypso system with lung cancer patient trajectory data. Int J Radiat Oncol Biol Phys 2005;63:S26-7. [Crossref]
- van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol 2004;14:52-64. [Crossref] [PubMed]
- Whyte RI, Crownover R, Murphy MJ, et al. Stereotactic radiosurgery for lung tumors: Preliminary report of a phase I trial. Ann Thorac Surg 2003;75:1097-101. [Crossref] [PubMed]
- Jaffray DA, Drake DG, Moreau M, et al. A radiographic and tomographic imaging system integrated into a medical linear accelerator for localization of bone and soft-tissue targets. Int J Radiat Oncol Biol Phys 1999;45:773-89. [Crossref] [PubMed]
- Jaffray DA, Siewerdsen JH, Wong JW, et al. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys 2002;53:1337-49. [Crossref] [PubMed]
- Higgins J, Bezjak A, Franks K, et al. Comparison of spine, carina, and tumor as registration landmarks for volumetric image-guided lung radiotherapy. Int J Radiat Oncol Biol Phys 2009;73:1404-13. [Crossref] [PubMed]
- Sterzing F, Kalz J, Sroka-Perez G, et al. Megavoltage CT in helical tomotherapy—clinical advantages and limitations of special physical characteristics. Technol Cancer Res Treat 2009;8:343-52. [Crossref] [PubMed]
- Bissonnette JP, Purdie TG, Higgins JA, et al. Cone-beam computed tomographic image guidance for lung cancer radiation therapy. Int J Radiat Oncol Biol Phys 2009;73:927-34. [Crossref] [PubMed]
- Wang Z, Nelson JW, Yoo S, et al. Refinement of treatment setup and target localization accuracy using three-dimensional cone-beam computed tomography for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2009;73:571-7. [Crossref] [PubMed]
- Rosenzweig KE, Amols H, Ling C. New radiotherapy technologies. Semin Surg Oncol 2003;21:190-5. [Crossref] [PubMed]
- Sonke JJ, Zijp L, Remeijer P, et al. Respiratory correlated cone beam CT. Med Phys 2005;32:1176-86. [Crossref] [PubMed]
- Bissonnette JP, Franks KN, Purdie TG, et al. Quantifying interfraction and intrafraction tumor motion in lung stereotactic body radiotherapy using respiration-correlated cone beam computed tomography. Int J Radiat Oncol Biol Phys 2009;75:688-95. [Crossref] [PubMed]
- Korreman S, Persson G, Nygaard D, et al. Respiration-correlated image guidance is the most important radiotherapy motion management strategy for most lung cancer patients. Int J Radiat Oncol Biol Phys 2012;83:1338-43. [Crossref] [PubMed]
- Knap MM, Hoffmann L, Nordsmark M, et al. Daily cone-beam computed tomography used to determine tumour shrinkage and localisation in lung cancer patients. Acta Oncol 2010;49:1077-84. [Crossref] [PubMed]
- Kwint M, Conijn S, Schaake E, et al. Intra thoracic anatomical changes in lung cancer patients during the course of radiotherapy. Radiother Oncol 2014;113:392-7. [Crossref] [PubMed]
- Møller DS, Khalil AA, Knap MM, et al. Adaptive radiotherapy of lung cancer patients with pleural effusion or atelectasis. Radiother Oncol 2014;110:517-522. [Crossref] [PubMed]
- van Elmpt W, Öllers M, van Herwijnen H, et al. Volume or position changes of primary lung tumor during (chemo-) radiotherapy cannot be used as a surrogate for mediastinal lymph node changes: The case for optimal mediastinal lymph node imaging during radiotherapy. Int J Radiat Oncol Biol Phys 2011;79:89-95. [Crossref] [PubMed]
- Lavoie C, Higgins J, Bissonnette J, et al. Volumetric image guidance using carina vs spine as registration landmarks for conventionally fractionated lung radiotherapy. Int J Radiat Oncol Biol Phys 2012;84:1086-92. [Crossref] [PubMed]
- Lim G, Bezjak A, Higgins J, et al. Tumor regression and positional changes in non-small cell lung cancer during radical radiotherapy. J Thorac Oncol 2011;6:531-6. [Crossref] [PubMed]
- Higgins J, Bezjak A, Hope A, et al. Effect of image-guidance frequency on geometric accuracy and setup margins in radiotherapy for locally advanced lung cancer. Int J Radiat Oncol Biol Phys 2011;80:1330-7. [Crossref] [PubMed]
- Yeung AR, Li J, Shi W, et al. Optimal image-guidance scenario with cone-beam computed tomography in conventionally fractionated radiotherapy for lung tumors. Am J Clin Oncol 2010;33:276-80. [PubMed]
- Corradetti MN, Mitra N, Millar LPB, et al. A moving target: Image guidance for stereotactic body radiation therapy for early-stage non-small cell lung cancer. Pract Radiat Oncol 2013;3:307-15. [Crossref] [PubMed]
- Diwanji TP, Mohindra P, Vyfhuis M, et al. Advances in radiotherapy techniques and delivery for non-small cell lung cancer: Benefits of intensity-modulated radiation therapy, proton therapy, and stereotactic body radiation therapy. Transl Lung Cancer Res 2017;6:131. [Crossref] [PubMed]
- Giaddui T, Chen W, Yu J, et al. Establishing the feasibility of the dosimetric compliance criteria of RTOG 1308: Phase III randomized trial comparing overall survival after photon versus proton radiochemotherapy for inoperable stage II-IIIB NSCLC. Radiat Oncol 2016;11:66. [Crossref] [PubMed]
- Liao ZX, Lee JJ, Komaki R, et al. Bayesian randomized trial comparing intensity modulated radiation therapy versus passively scattered proton therapy for locally advanced non-small cell lung cancer. J Clin Oncol 2016;34:abstr 8500.
- Kang M, Huang S, Solberg T, et al. A study of the beam-specific interplay effect in proton pencil beam scanning delivery in lung cancer. Acta Oncol 2017;56:531-40. [Crossref] [PubMed]
- Zhang Y, Huth I, Wegner M, et al. An evaluation of rescanning technique for liver tumour treatments using a commercial PBS proton therapy system. Radiother Oncol 2016;121:281-7. [Crossref] [PubMed]
- Chang JY, Zhang X, Knopf A, et al. Consensus guidelines for implementing pencil beam scanning proton therapy for thoracic malignancies on behalf of PTCOG thoracic and lymphoma subcommittee. Int J Radiat Oncol Biol Phys 2017;99:41-50. [Crossref] [PubMed]
- Bernatowicz K, Lomax A, Knopf A. Comparative study of layered and volumetric rescanning for different scanning speeds of proton beam in liver patients. Phys Med Biol 2013;58:7905. [Crossref] [PubMed]
- Dowdell S, Grassberger C, Sharp G, et al. Interplay effects in proton scanning for lung: A 4D monte carlo study assessing the impact of tumor and beam delivery parameters. Phys Med Biol 2013;58:4137. [Crossref] [PubMed]
- Santiago A, Jelen U, Ammazzalorso F, et al. Reproducibility of target coverage in stereotactic spot scanning proton lung irradiation under high frequency jet ventilation. Radiother Oncol 2013;109:45-50. [Crossref] [PubMed]
- Veiga C, Janssens G, Teng C, et al. First clinical investigation of cone beam computed tomography and deformable registration for adaptive proton therapy for lung cancer. Int J Radiat Oncol Biol Phys 2016;95:549-59. [Crossref] [PubMed]
- Paganetti H. Range uncertainties in proton therapy and the role of monte carlo simulations. Phys Med Biol 2012;57:R99. [Crossref] [PubMed]
- España S, Paganetti H. Uncertainties in planned dose due to the limited voxel size of the planning CT when treating lung tumors with proton therapy. Phys Med Biol 2011;56:3843. [Crossref] [PubMed]
- Taylor PA, Kry SF, Alvarez P, et al. Results from the imaging and radiation oncology core houston's anthropomorphic phantoms used for proton therapy clinical trial credentialing. Int J Radiat Oncol Biol Phys 2016;95:242-8. [Crossref] [PubMed]
- Maes D, Bowen S, Fung A, et al. Dose comparison between proton pencil beam and monte carlo dose calculation algorithm in lung cancer patients. Int J Radiat Oncol Biol Phys 2017;99. [Crossref]
- Lin L, Huang S, Kang M, et al. A benchmarking method to evaluate the accuracy of a commercial proton monte carlo pencil beam scanning treatment planning system. J Appl Clin Med Phys 2017;18:44-9. [Crossref] [PubMed]
- Parodi K, Paganetti H, Shih HA, et al. Patient study of in vivo verification of beam delivery and range, using positron emission tomography and computed tomography imaging after proton therapy. Int J Radiat Oncol Biol Phys 2007;68:920-34. [Crossref] [PubMed]
- Knopf A, Parodi K, Bortfeld T, et al. Systematic analysis of biological and physical limitations of proton beam range verification with offline PET/CT scans. Phys Med Biol 2009;54:4477. [Crossref] [PubMed]
- Polf JC, Peterson S, McCleskey M, et al. Measurement and calculation of characteristic prompt gamma ray spectra emitted during proton irradiation. Phys Med Biol 2009;54:N519-27.
- Polf JC, Peterson S, Ciangaru G, et al. Prompt gamma-ray emission from biological tissues during proton irradiation: A preliminary study. Phys Med Biol 2009;54:731. [Crossref] [PubMed]


