Prolonged pleural catheters in the management of pleural effusions due to breast cancer
Introduction
Malignancy related effusions are classified as malignant pleural effusions (MPE) and paramalignant pleural effusions (PMPE). MPE is a sign of an advanced or generalized tumor stage (1). MPE is identified based on the proof of malignant cells in the pleural fluid or tissue, and it is detected in most of the patients with malignant tumors (approximately 50%) (1,2). Effusions in which there is a malignancy but no direct infiltration of the pleura are named PMPE (3). Almost all malignant tumors in advanced stage may affect the pleura, and may thereby result in pleural effusion or pleural carcinosis (4). The second most common etiologic cause of MPE after lung cancer is breast cancer (5-7). Moreover, there is a close relationship between breast cancer and MPE, 2-11% of the patients with breast cancer develop MPE during the course of the disease (8,9). Approximately 80% of the patients with pleural recurrences develop MPE within five years of primary breast cancer surgery, whereas MPE is rare beyond ten years after the surgery (10,11).
The clinical presentations of pleural effusions due to breast cancer are similar to that of other other pleural effusions; they are characterized with progressive dyspnea, cough, and pleuritic type chest pain. In addition, a poor general condition and symptoms of breast cancer may be present. The clinical presentation is related to the size (width) of the effusion, time to development, and the physical condition of the patient (4). Physical examination may show decreased lung sounds and dullness on percussion.
The most common and easiest method for investigating the presence of a pleural effusion in breast cancer patients is a chest X-ray (posteroanterior and lateral). Pleural ultrasonography (USG) may be used for the diagnosis of smaller quantities. USG is also helpful in determining the site of thoracentesis, especially when there is a small amount of collection (4).
The first step after detection of pleural fluid collection is pleural drainage performed by diagnostic and therapeutic thoracentesis (12). However in pleural effusions following breast cancer, the pleural fluid often reaccumulates after simple aspiration (10). Reaccumulation of pleural effusion ipsilateral or contralateral to the site of previous drainage is named recurrent pleural effusion. MPEs due to breast cancer also develop recurrent pleural effusions (10). There are numerous management alternatives in recurrent MPE and PMPE, pleurodesis is the most commonly used. It can be often applied with pleural catheter, tube thoracostomy, and videothoracoscopy (13,14). Some studies have advocated the use of permanent pleural drainage catheters due their easy application and use; the method has advantages compared to repeated thoracenteses or pleurodeses, such as successful alleviation of symptoms, decreased mortality, lower costs, and decreased length of stay (7,15-17).
In our study, we evaluated 26 patients with recurrent pleural effusions caused by breast cancer who underwent pleural drainage with Jackson Pratt (JP) silicone drains® because of the easy application and also lower costs of these drains.
Methods
In this study 26 patients who developed breast cancer related pleural effusions between January 2011 and July 2013, in whom no further treatments were considered and who accepted the JP drain treatment after being informed on other treatment alternatives were retrospectively analyzed. The patients included into the study had symptomatic or asymptomatic pleural effusions that did not respond to treatment or that recurred, and therefore received the indication for drainage. Patients diagnosed with pleural effusions underwent insertion of 10 F JP® catheters which could be applied in a tunneled manner easily under local anesthesia.
The patients were referred to our unit mostly from the medical oncology departments after detection of pleural effusion with radiologic studies including posteroanterior chest X-ray, chest tomography, ultrasound, and also laboratory studies.
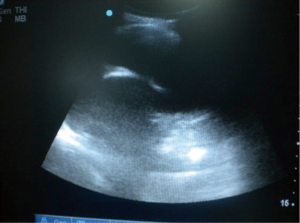
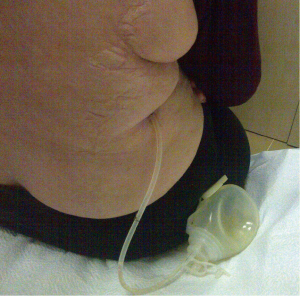
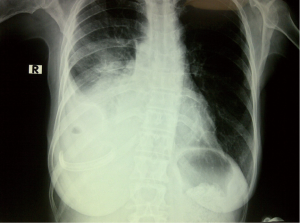
The insertion sites for the prolonged pleural catheters (JP® Drain) were determined with thoracic ultrasound prior to the procedure in order to find a position for easy access. The catheters were inserted from the lateral chest wall in all patients. Prior to the procedure, complete blood counts, bleeding and coagulation times, a PTT, and PT measurements were carried out. When the insertion sites could be determined by posteroanterior chest X-rays or chest CTs the catheters were inserted directly, in the remaining patients, especially in the presence of loculated effusions the insertion site was marked with a thoracic USG (Figures 1,2). Mean procedure time for catheter insertion was 15-20 minutes. The location of the catheter inside the thoracic cavity was confirmed with a postoperative chest X-ray (Figure 3). Daily drainage was restricted to 1,500 mL to avoid lung edema, drainage was also stopped when an abnormal cough disturbing to the patient began. Ambulatory patients in good general condition were discharged either home or to the referring unit after receiving instructions on the use of catheters. The catheters were removed once drainage decreased to less 50 mL/day and lung expansion was achieved.
The patients were evaluated with respect to age, sex, breast cancer cell type, and the period the catheter remained in place.
Results
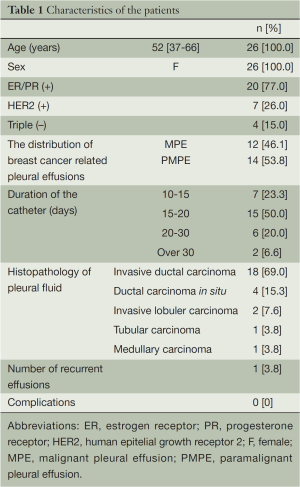
Thirty prolonged pleural catheters were inserted in 26 patients with MPE and PMPE due to breast cancer. All patients were female, mean age was 52. Twelve of the patients were diagnosed as MPE due to presence of tumor cells in the aspirate, the remaining 14 patients were diagnosed as PMPE due to their absence.
The most common histologic subtype of breast cancer was invasive ductal carcinoma, others were ductal carcinoma in situ, invasive lobular carcinoma, tubular carcinoma, and medullary carcinoma. The distribution of breast cancer according to cell type is shown on Table 1. Three patients underwent bilateral catheter insertion due to bilateral effusion. One patient developed a recurrent ipsilateral effusion 34 days after removal of the initial catheter (Table 1), and a new JP drain was inserted on the same side. This patient with recurrent effusion had invasive ductal carcinoma, and was diagnosed as PMPE.
Full table
One of the patients having invasive ductal carcinoma died while the catheter was in place. There were no preoperative or postoperative complications related to the procedure (Table 1).
Discussion
MPEs are associated with a poor prognosis. Median survival in breast cancer patients is 5 to 13 months after pleural fluid accumulation (7,10,18). Many patients with malignant effusions experience dyspnea, additional symptoms include weight loss, anorexia, malaise and fatigue, which negatively affect the quality of life. Therefore, management of MPEs is important to improve the quality of life of patients, inadequate management results in deterioration in respiratory function that can shorten the expected survival time.
Therapeutic thoracentesis is the initial approach for patients with respiratory symptoms including dyspnea. However, accumulation of pleural fluid usually recurs after a simple aspiration (10). There is currently no standard treatment approach in recurrent pleural effusions. Options include pleurodesis, pleuroperitoneal shunt, repeated thoracenteses, and pleural catheters.
Pleurodesis requires a chest tube and videothoracoscopic methods which prolong the length of stay in the hospital and increase the costs. Pleural catheters on the other hand cost less, require shorter hospitalizations, along with pleurodesis success rates reaching as high as 81% especially in MPE (19).
A pleuroperitoneal shunt can be used in recurrent pleural effusions, however a disadvantage with respect to practicality is requirement that the patient needs to use the pump, and approximately 1-2 cc liquid is transferred to the peritoneal cavity with each use of the pump (20).
The British Thoracic Society (BTS) report concluded that PPC was an effective alternative in the control of malignant effusions, minimized the length of stay and enabled avoidance of hospital admission (21). Davies et al. found that both talc pleurodesis and indwelling pleural catheters were effective treatments for relieving dyspnea and improving patients’ quality of life, but indwelling catheter were nor superior to talc pleurodesis for these outcomes (22). We preferred the PPCs due to their easy availability, lower costs, lower likelihood d of obstruction provided by the constant negative pressure, and practical use by the patients.
Some studies have advocated the use of prolonged pleural catheters in especially MPE. The most commonly used tunneled pleural catheter is Pleurx (Denver Biomaterials, Golden CO, USA), which is a 15.5 Fr diameter silicone tube that measures 24 inches long, has a perforated distal edge, and bears a valve at the proximal edge for drainage (7,16,19,23). It has been shown that the use of this catheter lowered the length of stay and costs (16,19).
Other simple and smaller diameter catheters may be also used in recurrent pleural effusions, however they have disadvantages such as a higher risk of extrusion compared to tunneled catheters and also inappropriateness for long term use. As a result of shorter stay in the body, they may have lower infection rates, which was measured to be around 5% in a previous study (19). There were no infections in our study.
The waiting period in the hospital is less for patients undergoing prolonged catheters, therefore all patients in this study were discharged on the same day without any complications. A previous study reported a 9.9% recurrence rate on the ipsilateral side (7). One of our patients (1/26, 3.8%) developed an ipsilateral collection one month after removal of the catheter.
Prolonged pleural catheters are suggested as the first line treatment in patients with trapped lung. The lung cannot expand in these patients and pleurodesis fails to prevent the recurrent pleural effusion. A decortication procedure will be a major invasive procedure, therefore such patients are suggested to undergo a prolonged pleural catheter or pleuroperitoneal shunt (20,24). None of the patients in our study had trapped lung syndrome.
An advantage of breast cancer compared to other malignancies is the efficacy of systemic treatment (cytotoxic and endocrine management) in decreasing the pleural fluid and treating dyspnea (25). All of our patients had received systemic treatment before and after catheter insertion. We believe that in our patients systemic treatment contributed to the high self sclerosis success rates provided by the catheter itself, and self sclerosis reached 96.2%.
Prolonged pleural catheter is a reliable and effective treatment method in recurrent pleural effusions (16,21). Self sclerosis rates as high as 81% were reported, with higher rates in gynecologic malignant effusions (19,26).
Our study has some limitations. First, it is a retrospective study and the sample size is small. Also, we did not perform a comparison with other alternatives regarding costs and length of stay.
The use of prolonged pleural catheters has become a widely accepted treatment recently for the management of especially MPE. We believe that in addition to its easy usage and success in providing effective pleurodesis, the JP® drain is effective in providing self sclerosis in breast cancer related pleural effusions.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kaifi JT, Toth JW, Gusani NJ, et al. Multidisciplinary management of malignant pleural effusion. J Surg Oncol 2012;105:731-8. [PubMed]
- Ludwig C, Stoelben E. Surgical therapy for malignant pleural effusions. Zentralbl Chir 2008;133:218-21. [PubMed]
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997;10:1907-13. [PubMed]
- Loddenkemper R. Management of malignant pleural effusions. Pneumologie 2005;59:120-35. [PubMed]
- Putnam JB Jr. Malignant pleural effusions. Surg Clin North Am 2002;82:867-83. [PubMed]
- Aydogmus U, Ozdemir S, Cansever L, et al. Bedside talc pleurodesis for malignant pleural effusion: factors affecting success. Ann Surg Oncol 2009;16:745-50. [PubMed]
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006;129:362-8. [PubMed]
- Apffelstaedt JP, Van Zyl JA, Muller AG. Breast cancer complicated by pleural effusion: patient characteristics and results of surgical management. J Surg Oncol 1995;58:173-5. [PubMed]
- Kreisman H, Wolkove N, Finkelstein HS, et al. Breast cancer and thoracic metastases: review of 119 patients. Thorax 1983;38:175-9. [PubMed]
- Fentiman IS, Millis R, Sexton S, et al. Pleural effusion in breast cancer. Cancer 1981;47:2087-92. [PubMed]
- Raju RN, Kardinal CG. Pleural effusion in breast carcinoma: analysis of 122 cases. Cancer 1981;48:2524-7. [PubMed]
- orcel JM, Light RW. Thoracentesis. PIER, American College of Chest Physicians, 2004. Accesed date: October 28, 2004. Available online: http://pier.acponline.org
- Patz EF Jr, McAdams HP, Erasmus JJ, et al. Sclerotherapy for malignant pleural effusions: a prospective randomized trial of bleomycin vs. doxycyline with small-bore catheter drainage. Chest 1998;113:1305-11. [PubMed]
- Lombardi G, Zustovich F, Nicoletto MO, et al. Diagnosis and treatment of malignant pleural effusion: a systematic literature review and new approaches. Am J Clin Oncol 2010;33:420-3. [PubMed]
- van den Toorn LM, Schaap E, Surmont VF, et al. Management of recurrent malignant pleural effusions with a chronic indwelling pleural catheter. Lung Cancer 2005;50:123-7. [PubMed]
- Putnam JB Jr, Light RW, Rodriquez RM, et al. A randomized comparision of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer 1999;86:1992-9. [PubMed]
- Demirhan O, Kasapoglu T, Ece F, et al. The use of Jackson-Pratt silicone flat drains as prolonged pleural catheters for the management of pleural effusions. J Thorac Disease 2013;5:265-9. [PubMed]
- Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of survival for patients with malignant pleural effusions. Chest 2000;117:79-86. [PubMed]
- Putnam JB Jr, Walsh GL, Swisher SG, et al. Outpatient management of malignant pleural effusion by a chronic indwelling pleural catheter. Ann Thorac Surg 2000;69:369-75. [PubMed]
- Ponn RB, Blancaflor J, D’Agostino RS, et al. Pleuroperitoneal shunting for intractablepleural effusions. Ann Thorac Surg 1991;51:605-9. [PubMed]
- Antunes G, Neville E, Duffy J, et al. BTS guidlines fort the management of malignannt pleural effusions. Thorax 2003;58 Suppl 2:ii29-38. [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [PubMed]
- Pollak JS, Burdge CM, Rosenblatt M, et al. Treatment of malignant pleural effusions with tunneled long-term drainage catheters. J Vasc Interv Radiol 2001;12:201-8. [PubMed]
- Pien GW, Gant MJ, Washam CL, et al. Use of an imporlantable pleural catheter for trapped lung syndrome in patients with malignant pleural effusion. Chest 2001;119:1641-6. [PubMed]
- Perrone F, Carlomagno C, De Placido S, et al. First-line systemic therapy for metastatic breast cancer and management of pleural effusion. Ann Oncol 1995;6:1033-43. [PubMed]
- Warren WH, Kim AW, Liptay MJ. Identification of clinical factors predicting Pleurx catheter removal in patients treated for malignant pleural effusion. Eur J Cardiothorac Surg 2008;33:89-94. [PubMed]