Transplantation of umbilical cord mesenchymal stem cells alleviates pneumonitis of MRL/lpr mice
Introduction
Systemic lupus erythematosus (SLE) is a common and potentially fatal autoimmune disease characterized by multi-organ injuries including renal, pulmonary, cardiovascular, neural, musculoskeletal, and cutaneous involvements. SLE can affect any organ at any stage, during the course of the disease, but the lungs are relatively involved late (1). Lung involvement can be sometimes the presenting feature of SLE in the form of pleuritis, pleural effusion, lupus pneumonitis or interstitial lung disease (ILD). Once the lungs are involved, there are always some other organs involved, such as glomerulonephritis, which represent SLE highly active. Acute lupus pneumonitis (ALP) or chronic ILD without an accurate treatment may lead to hypoxic respiratory failure and cause death in final. In 1999, Wang et al. (2) found that high-mobility group box chromosomal protein 1 (HMGB-1) can be released into the extracellular and mediate inflammatory responses, which was considered to be an important inflammation mediator of endotoxemia and sepsis. Recent studies have demonstrated that HMGB-1, actively secreted by macrophage/monocytes under inflammatory stimuli (3), was found to act as a proinflammatory cytokine in SLE. The presence of anti-HMGB1 antibodies correlates with disease activity in SLE patients (4). The induction of this proinflammatory cytokines may play a pathogenic role in the development of pneumonitis in MRL/lpr mice. Despite improved supportive care, aggressive immunosuppressive medical therapies, and new therapeutic interventions, a subset of SLE patients continue to suffer significant morbidity and mortality from active disease. Therefore, it is urgent to develop more effective therapy for SLE, especially for those who are refractory to treatment.
Mesenchymal stem cells (MSCs) are multipotent stem cells which are able to differentiate into a variety of cell types, including osteoblasts, chondrocytes, adipocytes, and myoblasts (5-7). These cells have been shown to have immunosuppressive properties and to reduce inflammation (8-11). Human MSCs suppress lymphocyte alloreactivity in vitro in mixed lymphocyte cultures through human leukocyte antigen-independent mechanisms (8). Previous studies showed that MSCs could inhibit lymphocyte proliferation induced by a variety of mitogens (11-13). Transplantation of ex vivo-expanded bone marrow MSCs (BM-MSCs) proved effective in treating acute graft-versus-host-disease (GVHD) by inhibiting T-lymphocyte function (14-16). MSCs, which can produce important growth factors and cytokines, have a strong propensity to ameliorate tissue damage in response to injury and disease. Relevant to this investigation, Huang et al. demonstrated that BM-MSCs could be transplanted into lung tissues of rats, and transformed into type II alveolar cells and was shown to prevent the development of pulmonary fibrosis (17). Sun et al. have reported that MSCs in patients with SLE grew much slower and showed senescence behavior compared with those in normal control patients (18). Based on these findings, we hypothesized that transplantation of allogeneic MSCs may be a potential therapeutic approach for SLE. Currently, BM-MSCs represent the major source of MSCs for cell therapy. However, aspiration of BM-MSCs is invasive, and the population and differentiation potential of BM-MSCs decrease significantly with age (19). Compared to BM-MSCs, umbilical cord-MSCs (UC-MSCs) may be collected without causing pain to the donors, and these cells have greater proliferative potential. Therefore, for allogeneic transplantation, UC-MSCs should be more promising than BM-MSCs. We have also found that UC-MSCs transplantation is effective in preventing the development of lupus-like nephritis in MLR/lpr mice (20). Then, what about lupus pneumonitis? Weather UC-MSCs have effect on the other hazardous complication of SLE or not?
In this study, our results indicated that UC-MSCs can relieve the extent of pulmonary injury in MLR/lpr mice, which may provide a new feasible measure for the management of lupus pneumonitis in SLE patients.
Materials and methods
Mice
Twenty-four female MRL/lpr mice (six weeks old), weighing 20.4±0.5 g (mean ± standard deviation, SD), were purchased from Shanghai SLAC Laboratory Animal Institute Co. Ltd. The mice were maintained in a specific pathogen-free animal facility of the Affiliated Hospital of Nantong University. The MRL/lpr mice were randomly divided into the following three groups (eight mice in each group): group 1 mice receiving 0.5 mL phosphate buffer saline (PBS) at 18 weeks of age (control); group 2 mice receiving transplantation of 1×106 UC-MSCs (UC-MSCT) once at 18 weeks of age; group 3 mice receiving multi-transplantation of 1×106 UC-MSCs (multi-UC-MSCT) at three consecutive weeks (18, 19, and 20 weeks of age); At 29 weeks of age, the mice were sacrificed and the lung tissue was collected. The experimental protocols conformed to the animal care guidelines of the China Physiologic Society and were approved by our Institutional Animal Research Committee.
MSCs culture
UC was obtained from the Gynecology Department at Affiliated Hospital of Nantong University. Tissue collection for this study was approved by The Affiliated Hospital Ethics Committee and informed consent was obtained from newborns’ parents. The tissue was minced into 1-2 mm3 pieces, and the minced tissue was incubated with 0.075% collagenase type II (Sigma, St Louis, MO, USA) for 30 min and then with 0.125% trypsin (Gibco, Grand Island, NY, USA) for 30 min with gentle agitation at 37 °C. Cells from UC were plated at a density of 1×106 cells/cm2 in non-coated T-25 cell culture flasks (Becton Dickinson, San Jose, CA, USA). Growth medium (GM) consisted of Dulbecco’s modified Eagle’s medium with low glucose (Gibco) and 5% fetal bovine serum (FBS, HyClone, Logan, UT, USA), supplemented with 10 ng/mL vascular endothelial growth factor (Sigma), 10 ng/mL epidermal growth factor (Sigma), 100 U/mL penicillin and 100 mg/mL streptomycin (Sigma), and 2 mmol/L glutamine (Gibco). Cultures were maintained in a humidified atmosphere with 5% CO2 at 37 °C. The medium was replaced after three days. The medium was changed twice weekly thereafter. A cell monolayer formed within two weeks, consisting of homogeneous bipolar spindle-like cells in a whirlpool-like array. Flow cytometric analysis showed that the UC-derived cells were positive for CD29, CD44, CD105, and CD166, but negative for CD14, CD34, CD38, CD45, and HLA-DR. Once 60-80% confluence had been reached, adherent cells were re-plated at a density of 1×104/cm2 in UC-MSCs growth medium (UC-GM) for expansion. After passage 3, cells were used for transplantation. Flow cytometric analysis was performed on passage 2.
Histopathological analysis
To assess pathologic lung changes after MSCs transplantation, the left lungs were cut into small pieces and fixed in 10% formalin for 24 h at 4 °C. Paraffin sections (4 mm) were stained with hematoxylin and eosin (HE) and Masson. The severity of airtube and vascellum injury was evaluated in a blinded manner by histologic examination of the sectioned lungs. Results were expressed according to the assay of Hasegawa et al. (21). The perivascular and peribronchiolar infiltrates were scored on the basis of histopathological findings: 0, normal; 1, less than three cell layers; 2, three to six cell layers; or 3, more than six layers. The index of perivascular lesion was indicated as the sum of all the scores per section divided by the number of all vessels per section. The index of peribronchiolar lesion was indicated as the sum of all the scores per section divided by the number of all bronchioli per section. The infiltrates in alveolar areas in high-power fields (×400 magnification) were scored as follows: 0, no infiltrating mononuclear cells; 1, less than 10 infiltrating cells; 2, less than 20 infiltrating cells; or 3, more than 20 infiltrating cells. The alveolar lesions index was indicated as the mean value of 20 random fields per section. The sections were evaluated by one of us, who was blinded to the treatment given.
RNA isolation and real-time quantitative PCR
To investigate the production of HMGB-1 in lung after the MSCs treatment, total RNA was extracted from pulmonary epithelial cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s recommendations. The production of HMGB-1 mRNA in lung was quantified by real-time quantitative polymerase chain reaction (PCR) using the TaqMan PCR MASTER MIX kit (Applied Biosystems, Foster City, CA, USA). The production of mRNA was determined and normalized to the expression of the internal housekeeping gene GAPDH. Primer and probe sequences are described as follows: HMGB-1 (359 bp): forward, 5'-ATGTTCTGCTCCTTACC-3', and reverse, 5'-AGTTTATCCGCTTTCC-3'.
Immunohistochemistry, western blot analysis
To detect HMGB-1 expression, lungs were snap-frozen in optional cutting temperature solution (OCT) compound (Sakura, Osuka, Japan) and cut into 5 μm pieces. Sections were analyzed by the avidin-biotin-peroxidase method, using biotin-labeled goat anti-murine HMGB-1 polyclonal antibody (Santa Cruz, CA, USA). Preimmune biotin-labeled goat serum served as a negative control. Analysis with monoclonal antibody (mAb) against human nuclei (MAB1281, Chemicon International) was performed following the manufacturer’s instructions to detect UC-MSCs in kidneys of mice treated with UC-MSCs. For western blot analysis, lung homogenates were blotted with anti-mouse HMGB-1 antibodies (Santa Cruz, CA, USA). Band detection was conducted using an enhanced chemiluminescence (ECL) detection system (Amersham Biosciences, Piscataway, USA).
Statistical analysis
Quantitative data were expressed as mean ± SD. SPSS 11.0 software was used for statistical analysis. The single-factor analysis of variance (ANOVA) was used for the comparison among multiple sample means. We considered P
Results
UC-MSCs transplantation alleviates pneumonitis of MRL/lpr mice
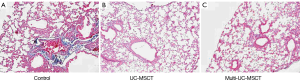
As mentioned above, there were three groups in the present study: control, UC-MSCT and multi-UC-MSCT. Two mice died respectively at 26 and 28 weeks of age in the control, which were also included in the following analysis. In control group, MRL/lpr mice showed typical interstitial lung disorders according to histopathology such as the perivascular and peribronchiolar focal aggregation of lymphocyte and mononuclear cell (Figure 1A). Besides the inflammatory cell infiltration, vascular congestion and edema were found in pulmonary interstitial. We found that all the treatment groups showed much less inflammatory cell infiltration in comparison with control mice. The index of perivascular and peribronchiolar lesion and the severity of inflammatory cell infiltration were much slighter (Figure 1B, Table 1). Further histological analysis demonstrated that the lungs from the treatment groups showed reduced deposition of collagen (Figure 2). In addition, the degree of lung injury in the multi-UC-MSCT group was significantly lower than that in the UC-MSCT. These findings suggest that UC-MSCT is a superior therapeutic approach for treating pneumonitis of MRL/lpr mice. Multi-infusion of UC-MSCs may enhance their effects.
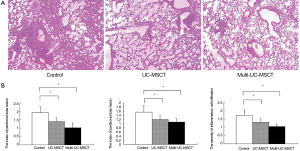

Full table
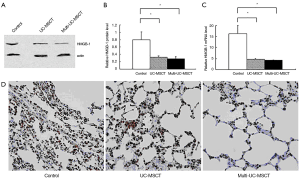
UC-MSCs transplantation decreases the expression of HMGB-1 in lung of MRL/lpr mice
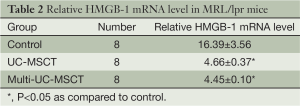
Recent studies have showed that HMGB-1 play an important role in the pathogenesis of SLE (4,22,23). We found that the expression of HMGB-1 protein in all the treatment mice was significantly lower than in control (Figure 3A,B; PFigure 3C, Table 2). Immunohistochemical staining for HMGB-1 showed marked intense staining in the control lungs. This positive staining was much weaker among all the treatment groups (Figure 3D). These results indicate that transplanting UC-MSCs is effective in the treatment of SLE pneumonitis, possibly by inhibiting HMGB-1 expression.
Full table
Discussion
SLE is sometimes a severe and thorny disease that often represents a therapeutic challenge because of its heterogeneous organ manifestations. Symptomatic pulmonary manifestations occur in 40% to 50% of the patients with SLE during the course of the disease (24). At autopsy, histological changes associated with SLE are found in almost all cases (25). Pneumonitis in SLE can be a severe and potentially life-threatening complication even despite current optimized therapy. The pulmonary manifestation of lupus is an important indicator of overall prognosis (26). Generally, pneumonitis in SLE patients will primarily be treated with glucocorticoids, cytotoxic and immunoregulatory agents (27). Alveolar haemorrhage is seen as an indication for additional plasma exchange (28-30). Rituximab has also been reported to provide beneft in these conditions (31-33) and infliximab has suggested effective to ILD which is refractory to cyclophosphamide (30). Though, the severe and life-threatening pulmonary manifestations in SLE, such as the acute course of lupus pneumonitis and the more smouldering course of ILD, represent a therapeutic challenge.
Previous studies about MSCs and ILD mainly concentrated in bleomycin (BLM) induced lung injury. MSCs can homing to and locate in the damaged lung tissue (34-36). After transplanting MSCs from male BALB/c rats to female C57BL/6 rats which have been caused lung damage by BLM, Ortiz et al. have found that donor derived MSCs can be settled at the site of injury of pulmonary receptors induced by BLM, and showed the epithelial like morphology, and can reduce the degree of inflammation and collagen deposition (35). Studies from Zhao et al. showed that MSCs in rat lung tissue damaged of BLM to differentiate into alveolar epithelial cells and bronchial epithelial cells (36). Rojas et al. (34) has also found that the protective mechanism of MSCs on lung injury induced by BLM is related to not only the MSCs differentiation to specific phenotype of lung cell, but also the increasing granulocyte colony stimulating factor (G-CSF) and granulocyte macrophage colony stimulating factor (GM-CSF) which promote its own stem cell mobilization, and the decreasing release of inflammation factors involved in.
In the present study, we have demonstrated that transfusions of xenogeneic UC-MSCs significantly attenuate the severity of lung injury in MRL/lpr mice. There were significant differences in the airtube and vascellum injury levels between the treatment and control groups. Light microscopic examination of the lung tissues showed that the improvement of pulmonary pathology correlates well with reduced deposition of collagen and the infiltration of the interstitial inflammatory cell. It is of interest that three transfusions provided more significant reduction in the above-mentioned disease activity manifestations.
HMGB-1, originally characterized as a nuclear DNA-binding protein to be a regulator of transcription, has also been described to have an extracellular role when it is involved in cellular activation and proinflammatory responses (3,37-39). Monocytes and macrophages stimulated by LPS, TNF-α, or IL-1, secrete HMGB-1 (40,41). Addition of HMGB-1 to monocytes, macrophages, or neutrophils in culture induces the release of proinflammatory cytokines, including TNF-α, IL-1α, IL-1β, IFN-γ, MIP-2, and IL-8 (40-43). It can also activate the endothelial cells, increasing the expression of vascular cell adhesion molecule and cell adhesion molecules (44), which leads to the accumulation of inflammatory cells in the vascular wall to produce vasculitis. HMGB-1 has been considered as a new pattern of inflammatory factor.
HMGB-1 has also been shown to act as an endogenous immune adjuvant by activating antigen-presenting cells (including dendritic cells and macrophages), through the receptor of advanced glycation end products (RAGE) and possibly toll-like receptor 2 and 4 mechanisms (45,46). Interestingly, it was recently shown that HMGB-1 and RAGE mediated TLR9-dependent activation of plasmacytoid dendrite cells by DNA-containing ICs (47). Thus, HMGB-1 plays an important role on the function of the immune system.
SLE is an autoimmune disease, whose pathological basis is vasculitis. Anti-HMGB-1 antibody was found in SLE patients (22). Popovic et al. colleagues have found high amounts of extracellular HMGB-1 in skin lesions of lupus (23). Deocharan and his colleagues found that immunization of non-autoimmune mice with a-actinin induced strong anti-nuclear antibody (ANA) response, particularly against chromatin. Furthermore, kidney glomerular IgG deposition and proteinuria were present in a-actinin-immunized mice (48). All above indicated HMGB-1 had an important effect on the genesis and development of SLE.
Al-Mutairi et al. reported that proinflammatory cytokines (TNF-α, IFN-γ, IL-8, IL-6) were more prevalent in the serum of SLE patients with pulmonary involvement compared with those without pulmonary manifestations (49). And HMGB-1 is deeply involved in inflammation and immunity. Studies from Maria et al. showed that inhibition of HMGB-1 protects against pseudomonas aeruginosa pneumonia in cystic Fibrosis (50). In the present study, we showed that the expression of HMGB-1 in lung was significantly reduced in all the treated mice in comparison with that in control animals. Therefore, downregulation of HMGB-1 expression may be one of the mechanisms involved in the treatment of MRL/lpr mice pneumonitis by UC-MSCs.
In summary, our study has shown that infusion of UC-MSCs exerts a therapeutic effect in treating pneumonitis in MRL/lpr mice without obvious major side effects. UC-MSCs were able to improved pulmonary pathological injury, reduced inflammatory cell infiltration, and reduced HMGB-1 expression in MRL/lpr mice. The results demonstrate that UC-MSCs could effectively prevent the development of pneumonitis of SLE. However, it remains to be determined whether UC-MSCs transfusions will reverse progression of established pneumonitis in SLE. Nevertheless, our findings provide an impetus for further investigations of the treatment of SLE with allogeneic MSCs readily available from umbilical cords.
Acknowledgements
This work was supported by Natural Science Foundation of China (30971306); and Six big talent peak in Jiangsu province project (the seventh batch, 033); and Nantong social development project (NO: S2009023); and Nantong fourth period “226 high-level personnel training project” project.
Disclosure: The authors declare no conflict of interest.
References
- Kamen DL, Charlie S. Pulmonary manifestations of systemic lupus erythematosus. Clin Chest Med 2010;31:479-88. [PubMed]
- Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999;285:248-51. [PubMed]
- Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 2000;192:565-70. [PubMed]
- Hayashi A, Nagafuchi H, Ito I, et al. Lupus antibodies to the HMGB1 chromosomal protein: epitope mapping and association with disease activity. Mod Rheumatol 2009;19:283-92. [PubMed]
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974;17:331-40. [PubMed]
- Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp 1988;136:42-60. [PubMed]
- Prockop DJ. Marrow stromal cells as stem cells for nonhemato-poietic tissues. Science 1997;276:71-4. [PubMed]
- Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 2003;57:11-20. [PubMed]
- Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99:3838-43. [PubMed]
- Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005;105:2821-7. [PubMed]
- Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003;101:3722-9. [PubMed]
- Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003;75:389-97. [PubMed]
- Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006;107:367-72. [PubMed]
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815-22. [PubMed]
- Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008;371:1579-86. [PubMed]
- Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol 2006;84:413-21. [PubMed]
- Huang K, Wu XM, Wang XY, et al. The effect of marrow mesenchymal stem cell transplantation on pulmonary fibrosis in rats. Zhonghua Jie He He Hu Xi Za Zhi 2012;35:659-64. [PubMed]
- Sun LY, Zhang HY, Feng XB, et al. Abnormality of bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus 2007;16:121-8. [PubMed]
- Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev 2001;122:713-34. [PubMed]
- Gu Z, Akiyama K, Ma XL, et al. Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus 2010;19:1502-14. [PubMed]
- Hasegawa H, Inoue A, Muraoka M, et al. Therapy for pneumonitis and sialadenitis by accumulation of CCR2-expressing CD4+CD25+ regulatory T cells in MRL/lpr mice. Arthritis Research Therapy 2007;9:R15-27. [PubMed]
- Uesugi H, Ozaki S, Sobajima J, et al. Prevalence and characterization of novel pANCA, antibodies to the high mobility group non-histone chromosomal proteins HMG1 and HMG2, in systemic rheumatic diseases. J Rheumatol 1998;25:703-9. [PubMed]
- Popovic K, Ek M, Espinosa A, et al. Increased expression of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in skin lesions of patients with lupus erythematosus. Arthritis Rheum 2005;52:3639-45. [PubMed]
- Kao AH, Sabatine JM, Manzi S. Lung disease in lupus. In: Wells AU, Denton CP. eds. Pulmonary involvement in systemic autoimmune diseases. Elsevier, 2004:125-246.
- du Bois RM, Wells AU. The lungs and connective tissue diseases. In: Mason RJ, Broaddus VC, Murray JF, et al. eds. Textbook of respiratory medicine. Elsevier Saunders, 2005:1609-33.
- Abu-Shakra M, Urowitz MB, Gladman DD, et al. Mortality studies in systemic lupus erythematosus. Results from a single center. I. Causes of death. J Rheumatol 1995;22:1259-64. [PubMed]
- Brasington RD, Furst DE. Pulmonary disease in systemic lupus erythematosus. Clin Exp Rheumatol 1985;3:269-76. [PubMed]
- Erickson RW, Franklin WA, Emlen W. Treatment of hemorrhagic lupus pneumonitis with plasmapheresis. Semin Arthritis Rheum 1994;24:114-23. [PubMed]
- Santos-Ocampo AS, Mandell BF, Fessler BJ. Alveolar hemorrhage in systemic lupus erythematosus: presentation and management. Chest 2000;118:1083-90. [PubMed]
- Chang MY, Fang JT, Chen YC, et al. Diffuse alveolar hemorrhage in systemic lupus erythematosus: a single center retrospective study in Taiwan. Ren Fail 2002;24:791-802. [PubMed]
- Aringer M, Houssiau F, Gordon C, et al. Adverse events and efficacy of TNF-alpha blockade with infliximab in patients with systemic lupus erythematosus: long-term follow-up of 13 patients. Rheumatology (Oxford) 2009;48:1451-4. [PubMed]
- Nellessen CM, Poge U, Brensing KA, et al. Diffuse alveolar haemorrhage in a systemic lupus erythematosus patient successfully treated with rituximab: a case report. Nephrol Dial Transplant 2008;23:385-6. [PubMed]
- Pinto LF, Candia L, Garcia P, et al. Effective treatment of refractory pulmonary hemorrhage with monoclonal anti-CD20 antibody (rituximab). Respiration 2009;78:106-9. [PubMed]
- Rojas M, Xu J, Woods CR, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 2005;33:145-52. [PubMed]
- Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. PNAS 2003;100:8407-11. [PubMed]
- Zhao F, Li SQ, Zhang YF, et al. Differentiation of bone marrow mesenchymal stem cells in injured rat lung. Medical Journal of Chinese People’s Liberation Army 2007;32:131-3.
- Yamada S, Maruyama I. HMGB1, a novel inflammatory cytokine. Clin Chim Acta 2007;375:36-42. [PubMed]
- Rovere-Querini P, Capobianco A, Scaffidi P, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep 2004;5:825-30. [PubMed]
- Gardella S, Andrei C, Ferrera D, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 2002;3:995-1001. [PubMed]
- Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 2000;192:565-70. [PubMed]
- Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999;285:248-51. [PubMed]
- Park JS, Svetkauskaite D, He Q, et al. Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 2004;279:7370-7. [PubMed]
- Park JS, Arcaroli J, Yum HK, et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol 2003;284:C870-9. [PubMed]
- Lenz O, Contreras G. Treatment options for severe lupus nephritis. Arch Immunol Ther Exp (Warsz) 2004;52:356-65. [PubMed]
- Taguchi A, Blood DC, del Toro G, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 2000;405:354-60. [PubMed]
- Park JS, Gamboni-Robertson F, He Q, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol 2006;290:C917-924. [PubMed]
- Tian J, Avalos AM, Mao SY, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 2007;8:487-96. [PubMed]
- Deocharan B, Zhou Z, Antar K, et al. Alpha-actinin immunization elicits anti-chromatin autoimmunity in nonautoimmune mice. J Immunol 2007;179:1313-21. [PubMed]
- Al-Mutairi S, Al-Awadhi A, Raghupathy R, et al. Lupus patients with pulmonary involvement have a proinflammatory cytokines profile. Rheumatology Int 2007;7:621-30.
- Entezari M, Weiss DJ, Sitapara R, et al. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against pseudomonas aeruginosa pneumonia in cystic fibrosis. Mol Med 2012;18:477-85. [PubMed]


