Characteristics of progression to tyrosine kinase inhibitors predict overall survival in patients with advanced non-small cell lung cancer harboring an EGFR mutation
Introduction
Lung cancer is the leading cause of cancer-related deaths in the world, it is often diagnosed at a late stage and survival with traditional first-line chemotherapy platinum-based regimens is generally poor (1,2). Currently, molecular testing for patients with advanced-stage lung adenocarcinoma is a routine activity, which aims to define the most optimal treatment strategy (3,4). Prognosis for advanced stage disease has improved in the past 20 years with tyrosine kinase inhibitors (TKIs) in patients with epidermal growth factor receptor (EGFR) mutation, nevertheless, uncommon mutations are associated with poor prognosis (5,6). Non-small cell lung cancer (NSCLC) harboring EGFR-sensitizing mutations has a distinct biology with improved patient survival compared to other subtypes of lung cancer. Somatic mutations in EGFR have been demonstrated as the most important biomarker in predicting the clinical outcome for NSCLC patients treated with EGFR-TKIs (3,7). In randomized phase III trials, treatment of NSCLC patients who present an activating EGFR mutation with EGFR-TKIs was associated with a longer progression-free survival (PFS), higher radiographic response rates and improved quality of life when compared to treatment with standard first-line platinum-based chemotherapy, in addition to being cost effective (8-10). However, despite a dramatic initial response, almost all patients treated with EGFR-TKIs eventually develop acquired resistance to these drugs with a median time to disease progression of 10–14 months (5,8,9,11-15). The Response Evaluation Criteria in Solid Tumors (RECIST) has been used as a standard method for assessing response and defining progression in cancer patients receiving treatment (15). However, there is more evidence every day, which suggests that continuing treatment beyond radiographic progression could confer an advantage, especially in patients treated with targeted therapy or immunotherapy (16). Treatment guidelines for NSCLC recommend continued use of TKI and local therapy after disease progression in asymptomatic patients. This strategy mirrors experience in other tumors, particularly HER2-amplified breast cancer, in which it is also recommended continuing anti-HER2 therapy despite disease progression (17).
Several studies have evaluated the impact of patterns of progression after treatment with TKIs showing that continued EGFR-TKI treatment after progressive disease (PD) prolongs survival time compared to patients who switch to cytotoxic chemotherapy in a select population (18). Yang et al. (19) separated the pattern of progression after treatment with EGFR-TKI (erlotinib or gefitinib) as dramatic, gradual, and local progression. Their results show that patients with a gradual progression have better results in PFS and could represent a subset of patients who benefit from continuing TKIs rather than switch to cytotoxic chemotherapy. They also suggest that chemotherapy is better for the dramatic group with rapid tumor increment, and that patients with local progression benefit from continued therapy with TKIs and local treatment.
Using the criteria established by Yang to define models of progression to TKIs, we performed a retrospective analysis of patients treated with gefitinib, erlotinib or afatinib. The purpose of the current study was to evaluate patterns of progression in patients who received TKIs, and additionally assess the relationship between these progression patterns and clinical outcome in EGFR-mutated NSCLC patients.
Methods
Patients
We retrospectively collected clinical information from patients with histologically confirmed metastatic NSCLC, treated from June 2009 to December 2015 in three oncology centers of Latin America (Mexico, Costa Rica and Colombia). We examined patients with advanced NSCLC harboring activating EGFR mutation who were treated with EGFR-TKI in the first or second-line setting, and who showed radiological progression after treatment with EGFR-TKIs. We confirmed PD using the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1).
Progression patterns at the time of initial EGFR-TKIs failure
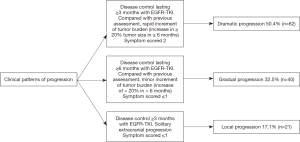
We used the criteria established by Yang (19) to define models of disease progression for TKI-treated NSCLC patients based on three clinical factors: the duration of disease control, evolution of tumor burden (rapid: vs. minor), and clinical symptoms. A detailed description is presented in Figure 1.
Statistical analysis
For descriptive purposes, continuous variables were summarized as arithmetic means and SDs, whereas categorical variables were expressed as frequencies and proportions. The χ2 or Fisher exact test were used for assessing the statistical significance of clinical and biochemical parameters. All continuous variables were dichotomized according with the median for PFS and OS analyses. PFS was defined as the time from first dose of EGFR-TKI to the first documentation of PD, or death from any cause or lost to follow up. Time to progression was defined as the time from first dose of EGFR-TKI to the first documentation of PD. Overall survival (OS) was calculated from first dose of EGFR-TKI to the last visit or death from any cause. The Kaplan-Meier method was used to estimate survival curves. The log-rank test was used to compare survival curves among patient groups. The multivariate Cox proportional hazards regression was used to evaluate independent prognostic factors associated with PFS or OS. All statistical tests were two-sided and P<0.05 was deemed to be statistically significant. SPSS software (version 22; SPSS; Chicago, IL, USA) was used for data analysis.
Results
Patient characteristics
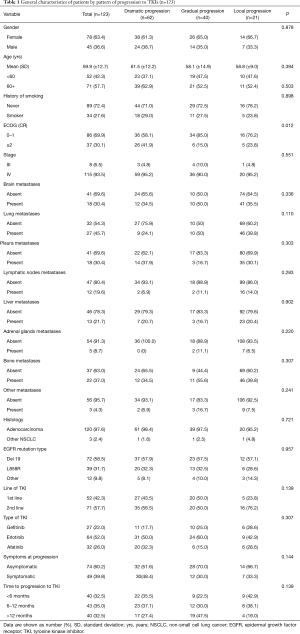
From June 2009 to December 2015, 123 patients with advanced NSCLC were analyzed at three Latin America (Mexico, Costa Rica and Colombia) oncology centers. Overall, 63.4% of patients were female and the mean age was 59.9 years (±12.7). Most patients were never-smokers (72.4%) and the Eastern Cooperative Oncology Group performance status (ECOG) was 0–1 in 69.9%. Histological examination revealed adenocarcinoma in 120 (97.6%) patients. All patients demonstrated an activating EGFR mutation, the main mutation detected was exon 19 deletion (58.5%), while the L858R mutation was detected in 39 patients (31.7%). These patients had been treated with TKIs in the first (42.3%) or second-line (57.7%) setting, following chemotherapy.
Patient characteristics according to clinical patterns of progression in relation to PFS
For all the studied population, 62 patients developed dramatic progression (50.4%), 40 patients had gradual progression (32.5%) and 21 patients presented local progression (17.1%). Demographic, clinical and pathological characteristics from patients were similar among the three progression pattern groups. However, patients with dramatic progression were more likely to have a worse ECOG performance status (≥2) in comparison with patients with gradual or local progression (P=0.012). At the time of progression, 39.8% of the patients were symptomatic, most symptomatic patients were in the group of dramatic progression (48.4%), while fewer patients were in the gradual progression (30.0%) or local progression (33.3%), however, this difference was not statistically significant (P=0.144) (Table 1).
Full table
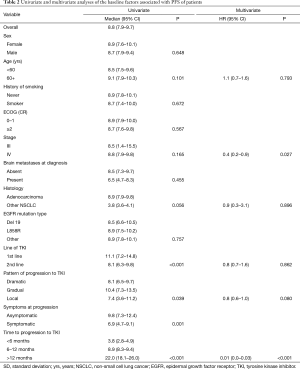
The median PFS was 8.8 months for all patients (95% CI, 7.9–9.7 months). The univariate analysis did not show differences in PFS by gender, age, smoking status, ECOG performance status, clinical stage, or EGFR mutation type (Table 2). Baseline factors associated to a longer PFS in the univariate analysis included line of TKI treatment [first (11.1, 95% CI: 7.2–14.8) vs. second-line (8.1, 95% CI: 6.3–9.8), P<0.001] and time to progression to TKI [<6 months (3.8, 95% CI: 2.8–4.9), 6-12 months (8.9, 95% CI: 8.3–9.4), >12 months (22.0, 95% CI: 18.1–26.0); P<0.001]. In the multivariate analysis the only two factors independently associated with PFS were disease stage (HR: 0.4, 95% CI: 0.2–0.9; P=0.027) and time to progression to TKI (HR: 0.01, 95% CI: 0.0–0.03; P<0.001) (Table 2).
Full table
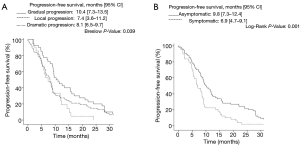
Factors at progression associated with a longer PFS included type of progression pattern [gradual (10.4, 95% CI: 7.3–13.5) vs. local (7.4, 95% CI: 3.6–11.2), vs. dramatic (8.1, 95% CI: 6.5–9.7), P=0.039] and symptoms at progression [asymptomatic (9.8, 95% CI: 7.3–12.4), vs. symptomatic (6.9, 95% CI: 4.7–9.1), P=0.001] (Figure 2A,B).
Patient characteristics according to clinical patterns of progression in relation to OS
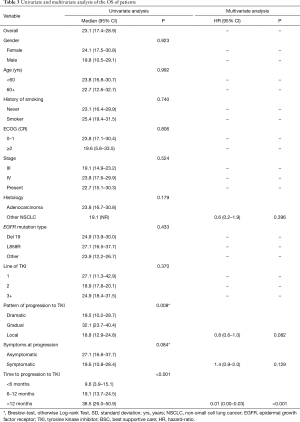
Median overall survival (OS) was 23.1 months (95% CI: 17.4–28.9). The univariate analysis did not show differences in OS by gender, age, smoking status, ECOG performance status, clinical stage, NSCLC histology, EGFR mutation type, nor by line or type of TKI (Table 3). Factors associated to a longer OS included pattern of progression [gradual progression (32.1, 95% CI: 23.7–40.4) vs. dramatic (19.5, 95% CI: 10.2–28.7) and local (18.8, 95% CI: 12.9–24.8 months), P=0.008] (Figure 3A), symptoms at the time of progression [asymptomatic (27.1, 95% CI: 16.6–37.7) vs. symptomatic (19.6 months, 95% CI: 10.8–28.4), P=0.084) (Figure 3B), and the time to progression to TKI [>12 months (38.5, 95% CI: 26.0–50.9), 6–12 months (19.1, 95% CI: 13.7–24.5) <6 months (9.6, 95% CI: 3.9–15.1), P<0.001] (Figure 3C). The latter factor was the only independent factor associated to a longer OS in the multivariate analysis (HR: 0.01, 95% CI: 0.00–0.03; P<0.001) (Table 3).

Full table
Additionally, a subgroup analysis which exclusively analyzed patients with exon 19 deletion revealed that a gradual pattern of progression to TKI was associated with a longer OS [gradual (32.8, 95% CI: 4.3–25.3), vs. local (32.4, 95% CI: 18.9–45.9) vs. dramatic (14.8, 95% CI: 4.3–25.3), P=0.008) (Figure 4A). However, this was not the case for patients harboring an exon21 L858R and other sensitizing mutations [gradual (22.4, 95% CI: 14.7–40.1) vs. local (14.5, 95% CI: 9.8–19.1), vs dramatic (22.7, 95% CI: 18.1–27.3), P=0.331) (Figure 4B).

Discussion
Molecular targeted therapies [erlotinib (5,20,21) gefitinib (8,9,11,22), afatinib (12,13,23,24)], have improved PFS in EGFR-positive lung cancer patients. Interestingly, epidemiological reports have shown a higher EGFR mutation prevalence in our study population (25,26). This can be explained by different exposure factors associated such as wood smoke exposure (27), high prevalence of chronic tuberculosis infection (28) and ethnicity (29). However, despite spectacular initial response to EGFR-TKIs, most patients eventually develop resistance to treatment and, subsequently, radiological and/or clinical progression.
In our study, in agreement with previous reports, we showed that patients with a dramatic pattern of progression were more likely to have a worse prognosis. This suggests that these patients may develop more aggressive resistance mechanisms. Hispanic patients undergoing re-biopsy show different mechanisms of acquired resistance, including the substitution of methionine for threonine at position 790 (T790M) in 47.1% of cases (14,30). However, PIK3CA and EGFR amplification is higher in Hispanics compared to other populations (14). Recently, Oya et al. described the progression pattern in patients with T790M mutation. In this study, response to EGFR-TKI, a duration of response greater than 6 months and single progression were associated with the development of T790M mutation (31). Our data showed that the median OS was significantly better in the group of patients with a sustained response which lasted for more than 12 months. This could be explained because a longer exposure to a TKI confers the time to develop a resistance mutation. Following this observation, previous studies have shown slower cell growth and favorable outcomes in patients with acquired resistance to EGFR-TKI associated to T790M mutations (32-35).
Suspending TKI therapy in patients who progress may not always be the best course, as it may result in accelerated tumor growth and precipitation of symptoms (36). Riely et al. (37) showed that patients who have responded for more than 6 months to erlotinib or gefitinib could benefit from continuing treatment despite documented progression of disease by RECIST criteria. Discontinuing treatment will result in worsening of lung cancer related symptoms, increase in tumor size and tumor fluorodeoxyglucose (FDG) uptake, a phenomenon described as “tumor flare”. In these patients TKI reintroduction produced stabilization or improvement in symptoms and reduction in tumor FDG uptake (37). However, it is important to dissect which patients would benefit from continued therapy.
Several studies have attempted to correlate the pattern of progression to EGFR-TKIs with clinical outcomes (38). Gandara et al. (39) proposed three subtypes of progression according to the site and mechanisms of acquired resistance: (I) central nervous system sanctuary PD, (II) oligo-PD and (III) systemic PD. In this classification oligo-PD was defined as new lesions, or growth of existing lesions, in a localized area (maximum four sites). However, it does not consider other clinical variables, or time to progression. In a Japanese study (40) which included 104 patients with EGFR mutated NSCLC treated with TKIs and evidence of PD, the oligo progression and asymptomatic status were significantly associated with clinical outcome after failure of EGFR-TKIs, regardless of subsequent treatment. However, the analysis included 9 patients with carcinomatous meningitis as oligo-progression. These patients are widely recognized for representing a subgroup of poor prognosis. Data from the univariate analysis of our study populations allows us to present two specific subgroups of patients with improved OS: patients with gradual progression and patients with a time to progression to TKI of at least 12 months. This last characteristic was the only statistically significant independent factor associated with a longer OS in the multivariate analysis (41).
In the AURA 3 trial, osimertinib showed to be more effective than combination platinum-based chemotherapy in patients with T790M-positive NSCLC after disease progression with first-line EGFR-TKI therapy (42), becoming a standard of treatment in this group of patients. However, although the benefit of osimertinib is overwhelming, half of the patients will not be candidates (T790M-negative) and others will not have access to the drug (43). Our data helps identify a subset of patients treated with TKIs who are likely to benefit from continuing treatment beyond progression, local ablative treatment and close surveillance, especially in countries where access to third-generation TKIs is limited. The ASPIRATION (Asian Pacific trial of Tarceva® as first-line in EGFR mutation) trial demonstrated the efficacy of first-line treatment with erlotinib, and showed that treatment continuation is feasible beyond progression. This is helpful in delaying treatment with chemotherapy, thus avoiding unnecessary toxicity, in patients with good response to TKI, longer time to progression or good ECOG at the time of PD (18). Therefore, it is expected that patients with slow progression might benefit from this strategy. Patients who have dramatic progression have rapid functional impairment, therefore, identification of this subgroup of patients is important to avoid delaying further treatment.
This is the largest reported series that evaluates the details of progression patterns and prognosis to treatment with EGFR-TKIs for advanced NSCLC in patients harboring an EGFR mutation. This work has inherent limitations due to its retrospective design, including the lack of a control arm and the small number of patients included, which might condition the sensibility of the multivariate analysis. However, future studies, which seek to evaluate TKI treatment beyond progression, might consider progression characteristics and patterns, in order to better identify patients who might reap more benefit from continuing TKI therapy.
Conclusions
Acquired resistance to TKIs is the common scenery for EGFR-mutated patients receiving targeted therapy. Our data suggests that the pattern of progression and time to TKI response are relevant factors to be considered at the time of progression to TKI, especially in countries with restrictions to molecular tests and limited health resource. These results are aimed at improving the selection of patients who are candidates for a therapeutic switch or re-challenge. However, validation of these results is required with randomized prospective clinical trials. Data from studies evaluating osimertinib conclude this is a feasible first-line treatment, and these concepts may be applied in patients treated with third-generation therapy in the first-line setting. These results would facilitate the development of therapeutic strategies beyond PD diagnosis after first-line EGFR-TKI treatment failure.
Acknowledgements
None.
Footnote
Conflicts of Interest: AFC has received grants from Roche, Boehringer Ingelheimm, Astra Zeneca and Pfizer, consulting fees from Roche, Boehringer Ingelheimm, Astra Zeneca, Pfizer, Merck Serono Foundation Medicine, MSD, BMS, payment for lectures from Roche, Boehringer Ingelheimm, Astra Zeneca, Pfizer, Merck Serono Foundation Medicine, MSD, BMS, and fees for expert testimony from Roche, Boehringer Ingelheimm, Astra Zeneca, Pfizer, Merck Serono Foundation Medicine, MSD, BMS. LC has participated on advisory boards by Astra Zeneca, and has received honoraria from Astra Zeneca for lectures in scientific meetings. OA has received payment for lectures from Boehringer ingelheim, Astra Zeneca, Merck and Lilly. All other authors have no competing interest to disclose. Preliminary results from this study were previously presented during the 17th World Conference on Lung Cancer–IASLC (4–7 December 2016, Vienna, Austria).
Ethical Statement: The protocol was approved by a central ethical and scientific committee in the National Cancer Institute in Mexico City (approval number 011/012/ICI, CB/678).
References
- Arrieta O, Guzman-de Alba E, Alba-Lopez LF, et al. National consensus of diagnosis and treatment of non-small cell lung cancer. Rev Invest Clin 2013;65 Suppl 1:S5-84. [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Cabanero M, Sangha R, Sheffield BS, et al. Management of EGFR-mutated non-small-cell lung cancer: practical implications from a clinical and pathology perspective. Curr Oncol 2017;24:111-9. [Crossref] [PubMed]
- Campos-Parra AD, Cruz-Rico G, Arrieta O. Personalized treatment in non-small cell lung cancer. Rev Invest Clin 2012;64:377-86. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Arrieta O, Cardona AF, Corrales L, et al. The impact of common and rare EGFR mutations in response to EGFR tyrosine kinase inhibitors and platinum-based chemotherapy in patients with non-small cell lung cancer. Lung Cancer 2015;87:169-75. [Crossref] [PubMed]
- Liao BC, Lin CC, Yang JC. First-line management of EGFR-mutated advanced lung adenocarcinoma: recent developments. Drugs 2013;73:357-69. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Arrieta O, Anaya P, Morales-Oyarvide V, et al. Cost-effectiveness analysis of EGFR mutation testing in patients with non-small cell lung cancer (NSCLC) with gefitinib or carboplatin-paclitaxel. Eur J Health Econ 2016;17:855-63. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Barron F, de la Torre-Vallejo M, Luna-Palencia RL, et al. The safety of afatinib for the treatment of non-small cell lung cancer. Expert Opin Drug Saf 2016;15:1563-72. [Crossref] [PubMed]
- Cardona AF, Arrieta O, Zapata MI, et al. Acquired Resistance to Erlotinib in EGFR Mutation-Positive Lung Adenocarcinoma among Hispanics (CLICaP). Target Oncol 2017;12:513-23. [Crossref] [PubMed]
- Nishino M, Jagannathan JP, Krajewski KM, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol 2012;198:737-45. [Crossref] [PubMed]
- Nishie K, Kawaguchi T, Tamiya A, et al. Epidermal growth factor receptor tyrosine kinase inhibitors beyond progressive disease: a retrospective analysis for Japanese patients with activating EGFR mutations. J Thorac Oncol 2012;7:1722-7. [Crossref] [PubMed]
- von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03-05 study. J Clin Oncol 2009;27:1999-2006. [Crossref] [PubMed]
- Park K, Yu CJ, Kim SW, et al. First-Line Erlotinib Therapy Until and Beyond Response Evaluation Criteria in Solid Tumors Progression in Asian Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer: The ASPIRATION Study. JAMA Oncol 2016;2:305-12. [Crossref] [PubMed]
- Yang JJ, Chen HJ, Yan HH, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer 2013;79:33-9. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Groen H, Arrieta OG, Riska H, et al. The global TRUST study of erlotinib in advanced non-small-cell lung cancer (NSCLC). J Clin Oncol 2008;26:19000. [Crossref]
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122-8. [Crossref] [PubMed]
- Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012;13:528-38. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Arrieta O, Cardona AF, Federico Bramuglia G, et al. Genotyping non-small cell lung cancer (NSCLC) in Latin America. J Thorac Oncol 2011;6:1955-9. [Crossref] [PubMed]
- Arrieta O, Cardona AF, Martin C, et al. Updated Frequency of EGFR and KRAS Mutations in NonSmall-Cell Lung Cancer in Latin America: The Latin-American Consortium for the Investigation of Lung Cancer (CLICaP). J Thorac Oncol 2015;10:838-43. [Crossref] [PubMed]
- Arrieta O, Campos-Parra AD, Zuloaga C, et al. Clinical and pathological characteristics, outcome and mutational profiles regarding non-small-cell lung cancer related to wood-smoke exposure. J Thorac Oncol 2012;7:1228-34. [Crossref] [PubMed]
- Luo YH, Wu CH, Wu WS, et al. Association between tumor epidermal growth factor receptor mutation and pulmonary tuberculosis in patients with adenocarcinoma of the lungs. J Thorac Oncol 2012;7:299-305. [Crossref] [PubMed]
- Arrieta O, Ramirez-Tirado LA, Baez-Saldana R, et al. Different mutation profiles and clinical characteristics among Hispanic patients with non-small cell lung cancer could explain the "Hispanic paradox". Lung Cancer 2015;90:161-6. [Crossref] [PubMed]
- Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res 2011;17:1169-80. [Crossref] [PubMed]
- Oya Y, Yoshida T, Kuroda H, et al. Association Between EGFR T790M Status and Progression Patterns During Initial EGFR-TKI Treatment in Patients Harboring EGFR Mutation. Clin Lung Cancer 2017;18:698-705.e2. [Crossref] [PubMed]
- Hata A, Katakami N, Yoshioka H, et al. Rebiopsy of non-small cell lung cancer patients with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor: Comparison between T790M mutation-positive and mutation-negative populations. Cancer 2013;119:4325-32. [Crossref] [PubMed]
- Li W, Ren S, Li J, et al. T790M mutation is associated with better efficacy of treatment beyond progression with EGFR-TKI in advanced NSCLC patients. Lung Cancer 2014;84:295-300. [Crossref] [PubMed]
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616-22. [Crossref] [PubMed]
- Tseng JS, Su KY, Yang TY, et al. The emergence of T790M mutation in EGFR-mutant lung adenocarcinoma patients having a history of acquired resistance to EGFR-TKI: focus on rebiopsy timing and long-term existence of T790M. Oncotarget 2016;7:48059-69. [Crossref] [PubMed]
- Chen HJ, Yan HH, Yang JJ, et al. Disease flare after EGFR tyrosine kinase inhibitor cessation predicts poor survival in patients with non-small cell lung cancer. Pathol Oncol Res 2013;19:833-8. [Crossref] [PubMed]
- Riely GJ, Kris MG, Zhao B, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res 2007;13:5150-5. [Crossref] [PubMed]
- Cha YK, Lee HY, Ahn MJ, et al. Survival outcome assessed according to tumor burden and progression patterns in patients with epidermal growth factor receptor mutant lung adenocarcinoma undergoing epidermal growth factor receptor tyrosine kinase inhibitor therapy. Clin Lung Cancer 2015;16:228-36. [Crossref] [PubMed]
- Gandara DR, Li T, Lara PN, et al. Acquired resistance to targeted therapies against oncogene-driven non-small-cell lung cancer: approach to subtyping progressive disease and clinical implications. Clin Lung Cancer 2014;15:1-6. [Crossref] [PubMed]
- Yoshida T, Yoh K, Niho S, et al. RECIST progression patterns during EGFR tyrosine kinase inhibitor treatment of advanced non-small cell lung cancer patients harboring an EGFR mutation. Lung Cancer 2015;90:477-83. [Crossref] [PubMed]
- Zhang Y, Sheng J, Kang S, et al. Patients with exon 19 deletion were associated with longer progression-free survival compared to those with L858R mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: a meta-analysis. PLoS One 2014;9:e107161. [Crossref] [PubMed]
- Mok TS, Wu YL, Papadimitrakopoulou VA. Osimertinib in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:1993-4. [Crossref] [PubMed]
- Raez LE, Santos ES, Rolfo C, et al. Challenges in facing the lung cancer epidemic and treating advanced disease in Latin America. Clin Lung Cancer 2017;18:e71-9. [Crossref] [PubMed]