Primary aortic sarcoma in arch and descending aorta: a case report and literature review
Introduction
Primary aortic sarcoma is a highly rare disease. Diagnoses are often delayed because of various clinical manifestations and no specific features in imaging examinations. Therefore, treatment to this kind of disease is usually challenging. Here, we present a case of primary intimal sarcoma and review literatures. Written informed consent was obtained from the patient’s family.
Case presentation
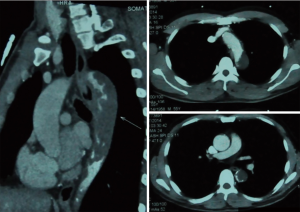
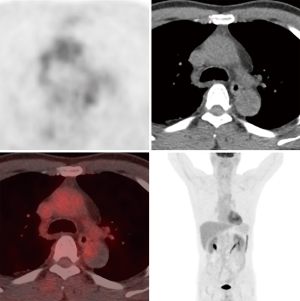
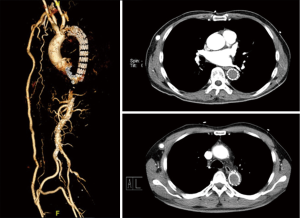
A 56-year-old man complaining of pain and numbness of bilateral lower extremities was transferred to our institute from another hospital. Cardiovascular risk factors were hypertension, hyperlipidemia and heavy smoking history. On examination, the ABI was 0.55. Chest contrast-enhanced computed tomography was performed and showed thrombus on the intersection of arch and descending aorta with severe luminal stenosis. Computed tomography angiography (CTA) of aorta further demonstrated atherosclerosis with multiple ulcerating plaques and thrombosis at the same location (Figure 1). Positron emission tomography/computed tomography (PET/CT) revealed no signs of increased intake in the lesion (Figure 2). Among the test of Protein C, Protein S and antithrombin III, activated Protein C resist, only Protein S was abnormal, presenting 179% (the reference was between 76% and 139%). The lupus anticoagulant (LA) presented with a value of 1.29 (the reference was <1.2). Both the tumor marker NSE (20.4 ng/mL) and CA-242 (25.3 U/mL) were beyond the normal range. For the last 2 months, he also had a history of deep vein thrombosis in his left lower limb and popliteal artery thrombosis in the right lower limb. Thrombus was initially suspected. Rivaroxaban was prescribed for 2 months. But no obvious change was seen in the descending aorta.
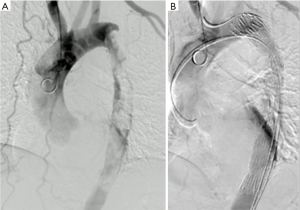
Considering that the lesion was clear and embolic events happened, a thoracic endovascular aortic repair (TEVAR) was performed to prevent recurrence. The patient was taken to the operating room timely. Access was accomplished via right femoral artery. Angiography was performed to confirm the lesion. A 90-cm sheath was introduced into the stenosis part of descending aorta. A few yellowish transparent tissue was sucked out through the sheath. A stent (COOK, 32–200 mm) was deployed along the filling-defect part (Figure 3A). Completion angiography demonstrated that the thoracic aorta and branches were patent (Figure 3B).
This patient was then transferred into the intensive care unit (ICU). Within the following 24 hours, he developed somnolence. An emergent head CT revealed multifocal low density image in the left temporal-occipital lobe and left thalamus. The patient finally returned well after 1-week supportive therapies in the ICU. One month later, the patient got well and was discharged home. Then he accepted additional radiation and chemotherapy in the outpatient clinic.
Histopathologic examination of the gelatinous tissue showed that spindle cells were loosely arranged. Immunohistochemical analysis presented positive for vimentin, indicating differentiation of muscular fibrocytes. Other immunohistochemical markers AE1/AE3, CD31, CD34, Desmin, SMA were all negative. The diagnosis of intimal sarcoma was finally made.
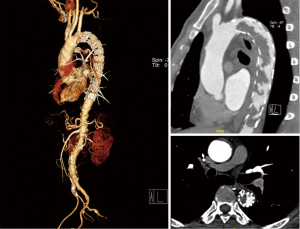
However, 6 months later the patient was readmitted to the hospital with complaints of intermittent claudication. Blood pressure reached up to 190/95 mmHg of bilateral upper limbs. The ABI was down to 0.37. CTA demonstrated a moderate-severe stenosis caused by thrombus in the lumen of the prior stent (Figure 4). As the patient and his family refused to undergo an en bloc resection, we decided to perform axillary bifemoral and femoro-femoral cross-over bypass surgeries to supply blood to the lower extremities. He got relieved and was discharged 10 days after the surgery.
He suffered a brain attack in the 22nd month without occlusion in the bridge vessel (Figure 5). In the 25th month, an epilepsy attacked to him and he got paralyzed. In the 37th month, he finally died of pulmonary infection.
Discussion
Primary aortic tumors are extremely rare. Since described in 1873 by Brodowski, aortic sarcomas have gradually gotten understood. Nearly 190 cases of aortic sarcomas have been reported. The classification of primary aortic tumors has several versions, which were introduced by different experts (1-3). In a clinicopathologic classification proposed by Wright et al. (2) the aortic sarcoma was divided into intimal and mural types. Based on the immunohistochemical pattern, another classification was introduced by Thalheimer et al. (3), including the intimal angiosarcomas, the intimal myofibroblastic sarcomas and the mural sarcomas. The intimal angiosarcomas can express endothelial-specific antigens such as CD31, WT-1, FLI-1.While the mesenchymal markers desmin and actin were positive in myofibroblastic sarcomas (3). According to a report described by Nishida et al. (4), some sarcomas of undifferentiated stromal cell origin can be positive for only vimentin. In this case, the immunohistochemical analysis revealed that only vimentin was positive and his microscopic appearance of gelatinous tissue showed that the spindle cells were loosely arranged. Thus, primary intimal sarcoma was diagnosed.
The tumors can locate in different parts of the aorta, from the descending thoracic aorta (34.9%) to the abdominal aorta (27.3%), the thoracoabdominal aorta (26.5%) and the aortic arch (11.3%) (5). Clinical manifestations of primary aortic tumors are variable. Signs and symptoms include clinical embolic events (33.9%), constitutional symptoms (32.1%) and claudication (28.5%), abdominal complaints (28.5%), aneurysm or pseudoaneurysm (26.7%), back pain (22.4%), hypertension (18.2%) and so on (6). The intimal-type shares approximately 80% of the primary aortic sarcomas, mainly manifesting aortic obstruction or peripheral emboli (7). Literature reported that the cerebral emboli was only associated with the arch tumor (6). According to multiple imaging in this patient, the space-occupying lesion can be seen in the arch and descending thoracic aorta. Both claudication and cerebral embolic events presented in this patient during the course.
Methods to detect the primary aortic tumor include computed tomography (CT), CTA, transesophageal echocardiography (TEE), magnetic resonance (MR), positron emission tomography (PET) and others (8-11). The patient in this study presented with embolic symptoms at his first time to the emergency ward in other hospital. Thrombosis of cardiac origin or aorta was highly suspected. Multiple imaging demonstrated the lesion location. Although his PET/CT showed no signs of increased uptake (SUVmax =2.3), the pathology finally revealed malignancy of the tumor. Thus, multiple imaging should be taken into consideration when embolic events happened. But lessons also should be learned that no signs of increased uptake doesn’t alway mean no malignant tumors.
Pathology is the gold standard for diagnosis. The rate of antemortem diagnosis is 73.9% (6). For the antemortem diagnosis, most are made by surgical resection. Intra-arterial biopsy was seldom made for the potential embolic complication. Ronaghi et al. (12) reported a primary aortic tumor through intra-aortic biopsy by using a 5.4-F biopsy forceps device. In this case, the specimens were sucked out through the sheath without the use of biopsy forceps.
Management of primary aortic tumors is surgical radical resection, palliative surgery and chemotherapy, radiation. Surgical radical resection is the most effective method for aortic sarcoma. Nicotera et al. (13) reported a mean survival time (17.1±21.6 months) in 16 patients with en bloc resection through literature review. At first, this lesion in this study was reckoned as thrombus. The patient and his family preferred minimally invaded surgery. Endovascular management was then adopted to prevent recurrent thrombus. Only several literatures (14-19) (Table 1) mentioned the use of stent for management. Zhang et al. (20) adopted a palliative surgery for a patient with an intimal sarcoma, in which axillary bifemoral and femoro-femoral cross-over bypass surgeries were performed. In this case, palliative surgery and chemoradiotherapy were both adopted. The palliative surgery in this case consisted of stent placement at the first surgery and axillary bifemoral and femoro-femoral cross-over bypass surgeries at the second operation to improve the blood supply of bilateral lower extremities.

Full table
Prognosis of aortic sarcoma is poor. A pooled analysis in 2014 by Rusthoven et al. (6) reported that the median survival for 122 patients was 11 months. The 3-year survival was 17.1% and the 5-year’s was 8.8%. It also showed that the absence of metastatic disease at diagnose, surgical resection and chemotherapy were associated with improved survival (P<0.001, P<0.001, P=0.02 respectively). In this case, we initially intended to take en bloc resection at his second admission in the 6th month of the first admission, as the pathology had already demonstrated aortic sarcoma. However, he and his family refused to do so but chose the alternative choice of bypass surgeries. In the 22nd month, a brain infarction attacked him and a head CT demonstrated this. But no more test was done to figure out whether it was a thrombus or metastasis. Three months after the brain infarction (the 25th month), he suffered an epilepsy and got paralyzed. In the 37th month, he finally died of pulmonary infection.
Conclusions
Multiple imaging approaches should be taken to detect the primary lesion when artery embolic events or intermittent claudication happen. Though aortic sarcoma is rare, it should be taken into consideration as one of the possible etiologies for thrombus in aorta. Palliative surgeries such as bypass, endovascular aortic repair may also be an alternative to treat aortic sarcoma.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient’s family.
References
- Salm R. Primary fibrosarcoma of aorta. Cancer 1972;29:73-83. [Crossref] [PubMed]
- Wright EP, Glick AD, Virmani R, et al. Aortic intimal sarcoma with embolic metastases. Am J Surg Pathol 1985;9:890-7. [Crossref] [PubMed]
- Thalheimer A, Fein M, Geissinger E, et al. Intimal angiosarcoma of the aorta: report of a case and review of the literature. J Vasc Surg 2004;40:548-53. [Crossref] [PubMed]
- Nishida N, Yutani C, Ishibashi-Ueda H, et al. Histopathological characterization of aortic intimal sarcoma with multiple tumor emboli. Pathol Int 2000;50:923-7. [Crossref] [PubMed]
- Chiche L, Mongredien B, Brocheriou I, et al. Primary tumors of the thoracoabdominal aorta: surgical treatment of 5 patients and review of the literature. Ann Vasc Surg 2003;17:354-64. [Crossref] [PubMed]
- Rusthoven CG, Liu AK, Bui MM, et al. Sarcomas of the aorta: a systematic review and pooled analysis of published reports. Ann Vasc Surg 2014;28:515-25. [Crossref] [PubMed]
- Lin SI, Su MI, Tsai CT. Primary Intimal Sarcoma of Thoracic Aorta Presenting as Hypertensive Crisis. Acta Cardiol Sin 2015;31:560-3. [PubMed]
- Kamran M, Fowler KJ, Mellnick VM, et al. Multimodality Imaging Approach towards Primary Aortic Sarcomas Arising after Endovascular Abdominal Aortic Aneurysm Repair: Case Series Report. Cardiovasc Intervent Radiol 2016;39:940-7. [Crossref] [PubMed]
- Choukroun EM, Labrousse LM, Madonna FP, et al. Mobile thrombus of the thoracic aorta: diagnosis and treatment in 9 cases. Ann Vasc Surg 2002;16:714-22. [Crossref] [PubMed]
- Hagspiel KD, Hunter YR, Ahmed HK, et al. Primary sarcoma of the distal abdominal aorta: CT angiography findings. Abdom Imaging 2004;29:507-10. [Crossref] [PubMed]
- Modi BP, Longo MJ, Kopf GS, et al. Surgical Management of Giant Descending Aortic Thrombus Detected by Transesophageal Echocardiography. Int J Angiol 2000;9:243-45. [Crossref] [PubMed]
- Ronaghi AH, Roberts AC, Rosenkrantz H. Intraaortic biopsy of a primary aortic tumor. J Vasc Interv Radiol 1994;5:777-80. [Crossref] [PubMed]
- Nicotera SP, Simosa HF, Campbell DR. Postoperative outcomes in intimal aortic angiosarcoma: A case report and review of the literature. J Vasc Surg 2009;50:186-9. [Crossref] [PubMed]
- Rusthoven C, Shames ML, Bui MM, et al. High-grade undifferentiated pleomorphic sarcoma of the aortic arch: a case of endovascular therapy for embolic prophylaxis and review of the literature. Vasc Endovascular Surg 2010;44:385-91. [Crossref] [PubMed]
- Ugurlucan M, Barburoglu M, Sayin OA, et al. Endovascular Treatment for Primary Aortic Angiosarcoma to Relieve Thoracic Aortic Stenosis. Ann Vasc Surg 2014;28:1799.e5-8.
- Ramjee V, Ellozy S. Aortic angiosarcoma masquerading as a thoracic aortic aneurysm. J Vasc Surg 2009;50:1477-80. [Crossref] [PubMed]
- Piffaretti G, Ferraro S, Carrafiello G, et al. Thoracic Endovascular Aortic Repair for Embolizing Total Occlusion of the Descending Aorta due to Aortic Sarcoma. Ann Vasc Surg 2017;39:286.e7-10. [Crossref] [PubMed]
- Peinado Cebrian J, Mestres Alomar G, Rodriguez Carvajal R, et al. Diagnosis and treatment of a symptomatic primary thoracic aortic tumor: endovascular exclusion to prevent recurrent embolization. Ann Vasc Surg 2014;28:492.e5-9. [Crossref] [PubMed]
- Ye ZX, Chen YX, You Y, et al. Bilateral renal artery occlusion, retroperitoneal mass and recurrent pseudoaneurysm in a patient. National Medical Journal Of China 2017;97:146-9.
- Zhang JL, Yang SM, Yao Q, et al. Palliative surgery for primary sarcoma in the abdominal aorta: A case report and review of the literature. Oncol Lett 2013;6:1738-40. [Crossref] [PubMed]