APRV for ARDS: the complexities of a mode and how it affects even the best trials
APRV is a mode of mechanical ventilation that has generated enough controversy to fuel a war. A major challenge has been the lack of randomized control studies on the application of APRV in patients with ARDS. All preexisting data did not address the question for which APRV was being promoted, that is, that APRV should be used as initial mode of mechanical ventilation for patients with ARDS. Multiple reports and studies in animals and humans have not helped answer this question. Not only is there paucity in the number of high quality trials in humans, but there is a lack of consistency on how APRV is applied (1,2).
Recently, Zhou and colleagues (3) published, perhaps the best (and first) evidence, on use of APRV on patients with ARDS. They studied 138 patients with a diagnosis of ARDS, and randomized them within 48 hours to conventional low tidal volume (LTV) ventilation with a low positive end-expiratory pressure (PEEP) strategy vs. APRV with a clearly defined implementation protocol. The study methods are transparent and clearly reported. Their primary outcome, ventilator free days was median 19 days (IQR 8–22 days) in APRV group vs. 2 days (IQR 0–15 days) in LTV group. This, along with several relevant secondary outcomes (better respiratory system compliance, improved gas exchange, less days in ICU) would make it sound as a straight hit: some will call it a home run.
We commend Dr. Zhou and colleagues (3) on their work, as this is the type of research that helps us move the field ahead. Clinicians often interpret a positive study as an affirmation of a treatment’s efficacy and effectiveness. However, the role of this editorial is to dissect this study in the context of current available literature, physiological concerns and technology issues. This is important, as we need to use the best available evidence, taken in the proper context, to make our clinical decisions.
External validity
The study by Zhou et al. (3) is an efficacy trial conducted at a single center, where the team was trained on use of APRV and followed a detailed protocol. The study was well powered to reach the primary outcome. The results demonstrate an impressive difference in the median days free of mechanical ventilation. The LTV group days on the ventilator (15 days) and ventilator free days (2 days) were worse than those reported in several large ARDS studies (Table 1). Why would this be the case? There are three important factors that may have affected the length of mechanical ventilation:
- The population studied had a higher proportion (58–69%) of ARDS from extra-pulmonary causes (sepsis, pancreatitis, trauma, and surgery) compared to other recent trials on ARDS (8,9). Although pulmonary vs. extra-pulmonary causes of ARDS have not shown to affect mortality (4,10), the response to positive pressure and ventilation strategies can be quite different depending on the cause, and this issue remains to be prospectively studied (11). This is important because of the relatively small number of patients in this study. Even with randomization, the groups were imbalanced in some baseline variables that could have affected the primary and secondary outcomes. For example, the LTV group had a higher incidence of pneumonia as a cause for ARDS along with more co-morbidities (COPD, renal dysfunction and malignancy), and a higher percentage of these patients were on vasopressors (68.7% vs. 56.3%). The presence of pre-existing conditions, shock and differing etiologies can obviously affect the outcomes of any mechanical ventilation strategy.
- The successful extubation rate in the LTV group was low, 38.8% (i.e., >60% of patients got re-intubated!). Failed extubation was not defined in the manuscript. Assuming the classic definition of extubation failure (need for re-intubation within 48–72 hours of extubation), a 60% extubation failure seems very high compared to the average reported in other studies (15%). Failed extubation is associated with increased mortality, ventilator days and, ICU/Hospital length of stay (12). The incidence of tracheostomy in the LTV group (29.9%) was higher than the 13% reported in Lung Safe study (8) which number was comparable to the 12.7% for the APRV group. Interestingly, the criteria used by the study team to perform a tracheostomy were related to airway patency, mental status, or physician expectations for prolonged MV. Failure to wean or prolonged mechanical ventilation, the most common cause for tracheostomy in ARDS, is not listed.
- The sedation on the LTV group was not titrated by the respiratory therapists as it was for the APRV group, thus potentially creating a treatment bias. The LTV group had a significantly higher need for sedation compared to APRV group, contrary to a previous study showing a trend towards increased sedation requirement for patient treated with APRV (13). Sedation, of course, is another important variable associated with prolonged mechanical ventilation. The depth of sedation and sedation protocols are associated with mechanical ventilation outcomes. How much, is yet to be determined, but different sedation practices can introduce unrecognized bias (14-16). More importantly, at least in the US, respiratory therapists do not titrate analgesics and sedatives. We commend Zhou et al. (3) on their respiratory therapists’ advanced training and privileging.
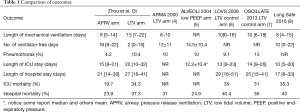
Full table
Thus, the results of this study should be taken with caution before generalizing to our patient population and clinical practice. It is a single center, efficacy study, with a small study population and a very strict research protocol. From the scientific standpoint, this study needs replication in larger populations and more centers before APRV can be considered as a standard of care.
Ventilator performance
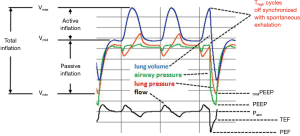
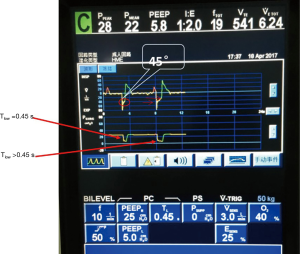
In terms of ventilator performance, this is a major area of caution for the APRV enthusiast. Zhou et al. (3) used a PB 840 to deliver Medtronic’s version of APRV. This ventilator has some particular issues that we need to consider. The authors carefully and appropriately measured the static compliance and resistance, and used this to calculate the time constant. They initially set the Tlow to 1–1.5 times the time constant. Then, they adjusted the Tlow to achieve a termination peak expiratory flow rate of ≥50%. The technical issues are: (I) the PB 840 does not measure the peak end expiratory flow rate or end (terminal) expiratory flow rate while on BiLevel ventilation, thus, calculations have to be made by trying to read on the ventilator screen the peak and terminal expiratory flow, which is difficult and can easily lead to errors. (II) The PB840 has a synchronization feature, which synchronizes the transition from Phigh to Plow with the expiratory phase of a spontaneous breath (if present) that occurs at the end of Thigh. This leads to a variable Tlow despite the fact that Tlow is preset (i.e., the synchronization feature overrides the setting). This phenomenon was described in a study of the BiLevel mode on the PB 84 ventilator (17). The study noted that the PB 840 ventilator is designed to cycle mandatory breaths (i.e., Phigh, Thigh) early if a spontaneous exhalation is detected in a synchronization window at the end of Thigh. As a result, the actual Tlow values (during simulated ventilation of an ARDS patient with spontaneous efforts) were not the ones set on the ventilator settings. The implication is that use of very short values for Tlow made the generation of total PEEP unpredictable. Tidal volumes were excessive (average 12.4 mL/kg) and total PEEP was not controllable using Tlow in this model (Figure 1). These results were actually confirmed in the supplemental material of the Zhou et al. study (Figure 2) demonstrate the variable Tlow.
Physiological premises
Finally, the issue with physiology; a major point in this study is the rapid improvement in gas exchange and respiratory system characteristics with APRV. We commend the authors for the precise methods they used to record these outcomes. We would like to make some points here. The first is related to the concept of “release” vs. “inflation” pressures, implying these are somehow unrelated. This notion is a misconception. It obscures the fact that APRV is identical to other modes in that the “releases” are nothing other than the last half of mandatory pressure controlled breaths. With every “re-pressurization”, the lung starts the first half of the mandatory breath, exposing the alveoli to volume increase and the risk of strain damage. Emphasizing only the exhalation portion of such a breath that APRV is less likely to injure the lung, and that tidal volume and pressure swings are of no consequence. On the contrary, the risk of injury is associated with that portion of the pressure-volume curve of the lungs on which the tidal volume occurs, which depends not only on the ventilator settings in APRV but on the patient’s inspiratory effort, and hence on the total change in transpulmonary pressure (18).
The second is to highlight the concern about the presence of spontaneous breaths during a Thigh. The presence of respiratory muscle pressure during Thigh exposes the lung to higher transpulmonary pressures. In the setting of heterogeneous lung injury, the potential for very high local transpulmonary pressures, raises the potential for more lung injury (19). Perhaps these swings can be ameliorated with some ventilator strategies (20,21), but the method has yet to be determined. Another important issue is the intensity and amount of minute ventilation supported by spontaneous breathes. Zhou et al. presented a novel strategy, in which the RTs controlled the level of sedation to maintain a specific level of respiratory effort. This strategy may minimize those transpulmonary pressure swings. Evidently, more studies are needed here.
The study by Zhou et al. provides images, respiratory characteristics, and gas exchange consistent with lung recruitment. This is likely due to the fact that the mean airway pressure was higher in the patients with APRV, as expected. The LVT group received the low PEEP ARDSnet table, and this plus lower I:E ratios led to lower mean airway pressures and worse markers of recruitment. Now, this begs two questions: if the LTV group had the same mean airway pressures, would the results be similar? And, does this matter? Literature on use of higher PEEP and thus, higher mean airway pressures, has shown improved gas exchange, and perhaps a decrease in rescue therapies, but no difference in ICU or hospital mortality (5,6,22). More importantly, not all patients respond similarly to PEEP, and we still work on trying to define what is the optimal level. With this in mind, we would caution readers on concluding overall success in the face of just improving gas exchange.
Finally, we must address the concept setting Tlow. As stated in the study by Zhou et al., “brief release phase (Tlow) could permit only partial lung volume loss at the release phase, avoid cyclic alveoli collapse, and provide dynamic homogeneity”. This statement has continued to permeate the literature. In recent years, very detailed studies (23,24) examining the effects of setting Tlow on APRV demonstrated that de-recruitment occurs very rapidly in animal lung models of ARDS. Actually, to maintain recruitment of the injured alveoli population requires a very short Tlow, less than 0.2 sec [which, by the way, not many ventilators can achieve (25) ]. Thus, APRV remains a mode with a potential to expose the lung to high transpulmonary pressures, cyclic de-recruitment and potentially high tidal volumes, along with the possibility of overconfidence in the face of improved oxygenation (26).
So overall, the article by Zhou and colleagues adds to the literature several items. First, it is the best described APRV protocol applied to patients with ARDS to date. Second, it describes a protocol where the respiratory therapists adjusted the level of sedation to achieve clearly delineated ventilation goals. Finally, it raises the potential for a strategy with APRV to be studied in a larger group. On the same breath we will highlight major concerns with APRV and ARDS that will have to be taken into account in any future trial. That the ventilator performance is not homogenous across platforms and software, that each ventilator has a different implementation of APRV and that we lack clear data on how to optimize ventilator settings for both the APRV and the control group. Furthermore, we emphasize that improvement in gas exchange is not equal to improved morbidity and mortality, that future studies should match sedation practices between experimental and control groups, and that we need to learn more about APRV and lung injury in spontaneous breathing.
Acknowledgements
None.
Footnote
Conflicts of Interest: Eduardo Mireles-Cabodevila and Siddharth Dugar have no conflicts of interest to declare. Robert L. Chatburn is a consultant for IngMar Medical and Drive Medical.
References
- Jain SV, Kollisch-Singule M, Sadowitz B, et al. The 30-year evolution of airway pressure release ventilation (APRV). Intensive Care Med Exp 2016;4:11. [Crossref] [PubMed]
- Rose L, Hawkins M. Airway pressure release ventilation and biphasic positive airway pressure: a systematic review of definitional criteria. Intensive Care Med 2008;34:1766-73. [Crossref] [PubMed]
- Zhou Y, Jin X, Lv Y, et al. Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive Care Med 2017;43:1648-59. [Crossref] [PubMed]
- Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327-36. [Crossref] [PubMed]
- Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:646-55. [Crossref] [PubMed]
- Ferguson ND, Cook DJ, Guyatt GH, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013;368:795-805. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Cavalcanti AB, Suzumura ÉA, Laranjeira LN, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017;318:1335-45. [Crossref] [PubMed]
- Agarwal R, Srinivas R, Nath A, et al. Jindal SK. Is the mortality higher in the pulmonary vs the extrapulmonary ARDS? A meta analysis. Chest 2008;133:1463-73. [Crossref] [PubMed]
- Pelosi P, D’Onofrio D, Chiumello D, et al. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl 2003;42:48s-56s. [Crossref] [PubMed]
- Krinsley JS, Reddy PK, Iqbal A. What is the optimal rate of failed extubation? Crit Care 2012;16:111. [Crossref] [PubMed]
- Maxwell RA, Green JM, Waldrop J, et al. A randomized prospective trial of airway pressure release ventilation and low tidal volume ventilation in adult trauma patients with acute respiratory failure. J Trauma 2010;69:501-10; discussion 511. [Crossref] [PubMed]
- Ouellette DR, Patel S, Girard TD, et al. Liberation From Mechanical Ventilation in Critically Ill Adults: An Official American College of Chest Physicians/American Thoracic Society Clinical Practice Guideline: Inspiratory Pressure Augmentation During Spontaneous Breathing Trials, Protocols Minimizing Sedation, and Noninvasive Ventilation Immediately After Extubation. Chest 2017;151:166-80. [Crossref] [PubMed]
- Aitken LM, Bucknall T, Kent B, et al. Protocol-directed sedation versus non-protocol-directed sedation to reduce duration of mechanical ventilation in mechanically ventilated intensive care patients. Cochrane Database Syst Rev 2015;1:CD009771. [PubMed]
- Kallet RH, Zhuo H, Yip V, et al. Spontaneous Breathing Trials and Conservative Sedation Practices Reduce Mechanical Ventilation Duration in Subjects With ARDS. Respir Care 2018;63:1-10. [Crossref] [PubMed]
- Haug K, Chatburn RL. Interactions among tidal volume, expiratory time, and total-PEEP in APRV. Respir Care 2014;59:OF12.
- Sasidhar M, Chatburn RL. Tidal volume variability during airway pressure release ventilation: case summary and theoretical analysis. Respir Care 2012;57:1325-33. [Crossref] [PubMed]
- Yoshida T, Torsani V, Gomes S, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med 2013;188:1420-7. [Crossref] [PubMed]
- Morais CCA, Koyama Y, Yoshida T, et al. High Positive End-Expiratory Pressure Renders Spontaneous Effort Non-Injurious. Am J Respir Crit Care Med 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Yoshida T, Roldan R, Beraldo MA, et al. Spontaneous Effort During Mechanical Ventilation: Maximal Injury With Less Positive End-Expiratory Pressure. Crit Care Med 2016;44:e678-88. [Crossref] [PubMed]
- Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010;303:865-73. [Crossref] [PubMed]
- Kollisch-Singule M, Jain S, Andrews P, et al. Effect of Airway Pressure Release Ventilation on Dynamic Alveolar Heterogeneity. JAMA Surg 2016;151:64-72. [Crossref] [PubMed]
- Kollisch-Singule M, Emr B, Smith B, et al. Airway pressure release ventilation reduces conducting airway micro-strain in lung injury. J Am Coll Surg 2014;219:968-76. [Crossref] [PubMed]
- Daoud EG, Chatburn RL. Comparing surrogates of oxygenation and ventilation between airway pressure release ventilation and biphasic airway pressure in a mechanical model of adult respiratory distress syndrome. Respir Investig 2014;52:236-41. [Crossref] [PubMed]
- Chatburn RL, Kallet RH, Sasidhar M. Airway Pressure Release Ventilation May Result in Occult Atelectrauma in Severe ARDS. Respir Care 2016;61:1278-80. [Crossref] [PubMed]


