Surgical treatment of chylothorax caused by lymphangioleiomyomatosis
Introduction
Lymphangioleiomyomatosis (LAM) is a rare disease. One of the most manifestations of the disease is chylothorax. A refractory chylothorax patient was admitted to our hospital. Biopsy of thoracic duct confirmed the diagnosis of LAM. We also explored multiple ligation in lower part of thoracic duct and the pleural infusions decreased markedly.
Case presentation
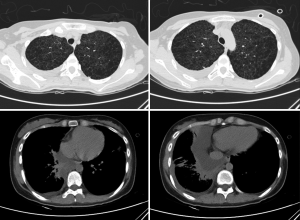
A 22-year-old female, with a history of pneumothorax two years ago, complained of shortness of breath for about one month. A CT scan in primary care hospital demonstrated right side pleural effusion and ovoid thin-walled cysts of both lungs, ranging from several millimeters to 2 cm (Figure 1). A closed drainage was done on the right thoracic cavity and drained chyle continuously ranging from 600 mL to 1,000 mL per day for about two months. Left side pneumothorax occurred during this hospitalization, and the patient underwent a left closed drainage. Thoracic drainage volume of chyle stabilized at about 600 mL each day for three weeks, although pleurodesis was performed.
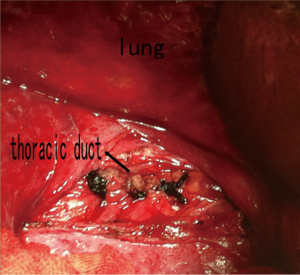
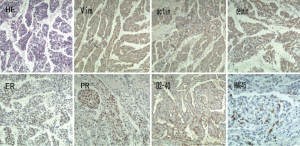
After admission to our hospital, the patient is forbidden to drink and eat and given total parenteral nutrition (TPN). The thoracic drainage volume didn’t decrease markedly. A new CT scan demonstrated effusion of right lung parenchyma. The arterial blood gas showed that pH 7.161, PO2 76.30 mmHg, PCO2 60.20 mmHg, SO2 89.60%, SB 17.60 mmol/L, AB 21.00 mmol/L and BE –8.2 mmol/L. Cancer Antigen 125 (CA-125) was up to 69.20 U/mL. Based on clinical features and CT scan, the patient is suspected with pulmonary LAM. Then the patient underwent video-assisted mini-thoracotomy (VAMT) on the right side. The thoracic duct was dilated and tortuous, just along the anterior right surface of the vertebral column in lower mediastinum, between the descending thoracic aorta and the azygos vein. Multiple ligation in lower part of thoracic duct and biopsy of the thoracic and right lung were done (Figure 2). Drainage volume of the right thoracic cavity decreased markedly from 550 to 50 mL. The drainage tube was removed on the 9th day after operation. Biopsy revealed LAM with positive immunohistochemical stainning for Vimentin (Vim), actin, desmin, estrogen receptor (ER), progestogen receptor (PR), D2-40, and the human melanoma black 45 (HMG45) (Figure 3).
Discussion
LAM is a rare progressive disease, occurring in about 3.4 to 7.8 per million women (1), that mainly affects productive female targeting the lung (PLAM), the lymph vessels. The kidney is often involved, showed as angiomyolipomas (AML’s) (2). It is characterized by progressive proliferating and infiltrating smooth muscle-like cells in the lung and lymph vessels. In this case, LAM cells can be seen both in the lung tissue and thoracic duct. LAM may occur sporadically or with Tuberous Sclerosis Complex (TSC)—an autosomal dominant genetic disease characterized by often manifests as autism, seizures and mental retardation (3). LAM manifestations include progressive dyspnea on exertion, recurrent pneumothorax and chylous pleural effusions and the patient presented all of them. An HRCT scan reveals diffuse ovoid thin-walled cysts, form millimeters to 3 cm, varying from scattered cysts to replacement of the whole pulmonary. In this case, cysts of the lung can be seen, but cyst wall is not clear. Ground glass opacities due to hemorrhage as well as pneumothorax and effusion sign may also be present. LAM is characterized by LAM cells organized in nodules along the pulmonary tissue and lymphatics and blood vessels. Two types of LAM cells can be seen, spindle-shaped cells and epithelioid cells. LAM cells express smooth muscle biomarkers-actin, Vim and desmin, as well as HMG45. In many cases, LAM cells also express estrogen and progesterone receptors (ER and PR). Podoplanin and D2-40 are often expressed by LAM cells. The patient expresses scattered HMG45 and all the other markers are positive except for podoplanin, and the diagnosis of LAM is clear.
The diagnosis standard of LAM is a tissue biopsy, though European Respiratory Society dose not recommend lung biopsy (2), for the differential diagnosis with other cystic lung disease, for example, emphysema, the common disease of lung.
At present, there is no effective treatment for LAM. Considering the expression of ER and PR, anti-estrogenic therapies are used by many medical centers, but no evidence are confirmed because of the lack of randomized controlled trial (4,5). The research of molecular pathways in the pathogenesis has led to targeted therapies, such as the use of sirolimus (or rapamycin), doxycycline, and letrozole. Sirolimes could inactivate mTOR complex which promote cell proliferation (6). Doxycycline could inhibit MMP’s, lower down the activity of enzymatic and cell proliferation (7). Letrozole could prevent the synthesis of estrogen (8). Lung transplantation is a valuable therapy for patients with end-stage LAM (9).
There is considerable controversy over management of chylothorax. Conservative treatment is first-line attempt with general success rate of 16% to 75% (10). The main indication for surgical intervention is failure of conservative management, but the correct timing for operation is not well defined. Early operation is recommended because the procedure is better tolerated by the patient with less nutritional and immunologic depletion, especially with the application of minimally invasive video-assisted thoracic surgery (VATS) (11). The most common surgical technique is thoracic duct ligation, sometimes mass ligation when thoracic duct is difficult to be identified. Suturing of thoracic duct leakage, pleurectomy and pleuroperitoneal shunt are also effective options in some cases.
Although the infiltration of LAM cells to lymphatic ducts is extensive, chylothorax patients without chyloperitoneum could benefit from multiple ligation of lower thoracic duct. Although lymph vessels are extensively involved, abdominal effusion didn’t occur after the thoracic duct was ligated. A possible reason is that the pressure of thoracic cavity is much lower and the thoracic duct is more susceptible of the disease.
A case of LAM diagnosed by thoracic duct biopsy has not been reported elsewhere in the English-language literature. Although surgical treatment of chylothorax caused by LAM is not recommended for diffuse infiltration of LAM cells and some patients would benefit from sirolimus treatment (12), multiple ligation in lower part of thoracic duct might be an effective option for selected patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Harknett EC, Chang WY, Byrnes S, et al. Use of variability in national and regional data to estimate the prevalence of lymphangioleiomyomatosis. QJM 2011;104:971-9. [PubMed]
- Johnson SR, Cordier JF, Lazor R, et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 2010;35:14-26. [PubMed]
- Moss J, Avila NA, Barnes PM, et al. Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex. Am J Respir Crit Care Med 2001;164:669-71. [PubMed]
- Taveira-DaSilva AM, Stylianous MP, Hedin CJ, et al. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest 2004;126:1867-74. [PubMed]
- Harari S, Cassandro R, Chiodini I, et al. Effect of a gonadotrophin-releasing hormone analogue on lung function in lymphangioleiomyomatosis. Chest 2008;133:448-54. [PubMed]
- McCormack FX, Inoue Y, Moss J, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med 2011;364:1595-606. [PubMed]
- Moses MA, Harper J, Folkman J. Doxycycline treatment for lymphangioleiomyomatosis with urinary monitoring for MMPs. N Engl J Med 2006;354:2621-2. [PubMed]
- Casanova A, Ancochea J. Lymphangioleiomyomatosis: new therapeutic approaches. Arch Bronconeumol 2011;47:579-80. [PubMed]
- Adachi K, Kurosawa S, Wagatsuma T, et al. Seventeen cases of lung transplantations for lymphangiomyoleiomatosis. Masui 2012;61:1239-44. [PubMed]
- Schild HH, Strassburg CP, Welz A, et al. Treatment options in patients with chylothorax. Dtsch Arztebl Int 2013;110:819-26. [PubMed]
- Misthos P, Kanakis MA, Lioulias AG. Chylothorax complicating thoracic surgery: conservative or early surgical management? Updates Surg 2012;64:5-11. [PubMed]
- Chachaj A, Drozdz K, Chabowski M, et al. Chyloperitoneum, chylothorax and lower extremity lymphedema in woman with sporadic lymphangioleiomyomatosis successfully treated with sirolimus: a case report. Lymphology 2012;45:53-7. [PubMed]