Early structural degeneration of Mitroflow aortic valve: another issue in addition to the mismatch?
Introduction
More than 200,000 surgical aortic valve replacements are performed yearly worldwide (1,2). This treatment has significantly evolved over the last 15 years, with a considerable increase in the use of aortic bioprosthesis relative to mechanical prosthesis in patients above the age of 65 years (3-5). Early structural valve degeneration (SVD) is a new emergent and underestimated problem, as we reported in a recent paper, based on a clinic and echocardiographic follow-up of 459 patients underwent aortic valve replacement with Mitroflow prosthesis (6). This problem has been addressed by several authors (7-14). Nevertheless, few histological data have been reported. Here we describe, two cases of early SVD at 2 and 4 years after their implantation in which we analyse microscopic aspects to validate the clinical and echocardiographic data widely argued in other studies.
Case presentation
First case (Figure 1)
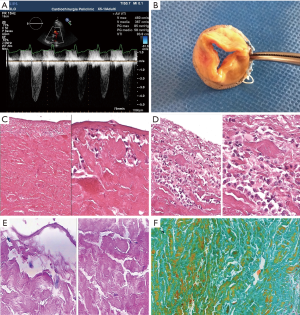
A 75-year-old woman is referred to Emergency Unit of “P. Giaccone” University Hospital, due to progressive shortness of breath on exertion without any clinical signs of infection. Specific and non-specific laboratory findings of inflammation are in the normal rage: erythrocyte sedimentation rate (ESR) is 5 mm/h (normal range, 1–7 mm/h); C-reactive protein (CRP) is 4 mg/L (normal value <6 mg/L); plasma viscosity (PV) is 1.1 (normal range, 1.05–1.30); procalcitonin is 0.08 (normal value 0.15 ng/mL); white blood cells (WBC) are 7.00×109/L (normal range, 4.00×109–11.00×109/L). Blood culture and urine culture are negative. She has previous graft operation and AVR (19-mm Mitroflow DL model) 2 years ago in the Cardio Surgery Unit of “P. Giaccone” University Hospital. She suffers from hypertension and diabetes. TTE shows a significantly increase of trans-prosthetic aortic valve gradients (mean transvalvular gradient 42 mmHg) and peak flow velocity (4.5 m/sec). TEE reveals a severe reduced mobility of the prosthesis cusps compatible with a SVD (Figure 1A). Coronary angiography shows no further progression of coronary artery disease and a good patency of the grafts. Redo operation is performed. The Mitroflow aortic valve is examined through a transverse aortotomy: the cuff of the prosthesis is covered with a thick intima, and the cusps are very stiff (Figure 1B). The prosthetic valve is removed and a 21-mm Mitroflow Crown PRT aortic valve is implanted. The patient is weaned from mechanical ventilation on the first day after surgery, and discharged on the 20th postoperative day.
Second case (Figure 2)

A 66-year-old man is referred to the Cardio-Surgery Unit of Tor Vergata University Hospital for congestive heart failure due to a paravalvular leak of the aortic prosthesis and dysfunction of the mitral prosthesis without any clinical signs of infection. He has a previous history of Staphylococcus Aureus endocarditis treated with antibiotic therapy and aortic and mitral valve replacement with a 25-mm Mitroflow DL model aortic valve and a 27-mm Edwards Perimount mitral valve 4 years ago in the Cardio Surgery Unit of Tor Vergata University. He suffers from hypertension and hypercholesterolemia. Specific and non specific laboratory findings of inflammation are in the normal rage: ESR is 4 mm/h (normal range, 1–7 mm/h); CRP is 5.5 mg/L (normal value <6 mg/L); PV is 1.18 (normal range, 1.05–1.30); procalcitonin is 0.04 (normal value 0.15 ng/mL); WBC are 10.00×109/L (normal range, 4.00×109–11.00×109/L). Blood culture and urine culture are negative. TEE reveals a severe reduced mobility of the aortic bioprosthesis with a moderate intraprosthesis regurgitation, a peak flow velocity above 4.7 m/sec and a mean transvalvular gradient above 45 mmHg compatible with a SVD, at the same time it shows a partial detachment of mitral valve prosthesis along the mitro-aortic continuity. An emergency redo operation is performed. The intraoperative findings confirm the TEE data: aortic prosthesis SVD and detachment of mitral valve prosthesis along the mitro-aortic continuity. The Mitroflow prosthesis and the aortic root are replaced with a composite mechanical valved conduit (23/26-mm CarboSeal, Sorin), the mitral prosthesis with a 27-mm Sorin Bi-Carbon prosthetic valve. At the intraoperative inspection, no signs of acute endocarditis (i.e., infected vegetations, laceration of cusp) are found on the Mitroflow prosthesis (Figure 2A), the leaflets appeared thickened and fibrotic with poor mobility. The mitral prostheses do not showed any signs of SVD or endocarditis, but because the annulus is partially detached along the mitro-aortic continuity, we decide to re-replaced the valve. At the weaning from cardiopulmonary bypass, the patient died of right ventricle failure and massive bleeding.
Microscopic findings
The valves are fixed in 10% neutral buffered formalin, photographed, macroscopically examined in detail for cusps tears and thickening, and the cusps are sectioned vertically from the base to the free margin for histological examination. In proximity of the stent, transverse sections parallel to the free margin are also taken to include the torn edge. Sections are embedded in paraffin, and 4-mm tick sections stained with Haematoxylin and Eosin, Alcian blue-PAS and Masson trichrome stains. Microscopic analysis shows a severe structural degeneration with collagen fragmentation and cusps thickening, most commonly at the free margin and in the para stent post region. The cusps are 2 to 3 times thicker than the pericardial tissue elsewhere. In particular, haematoxylin and eosin stain (Figure 1C,D and Figure 2B-E), show areas of collagen bundle separation and fiber fragmentation, fluid exudation, and a reactive inflammatory infiltration. Moreover, both valves demonstrate the presence of mononuclear and macrophage-like cells, as a diffuse layer on the out-flow surface and a patchy distribution on the in-flow surface. Distribution of inflammatory reaction seems to be associated to areas of collagen disruption. Alcian blue-PAS stains demonstrate extra-cellular matrix degeneration with mucopolysaccharides deposition and multiple cystic-like formation (Figures 1E,2F). Finally, Masson trichrome stains also shows areas of exudation and compact fibrin coating the leaflet surface (Figure 1F). In the second case, small and focal areas of calcification are also observed.
Discussion
In this paper, we report our experience on early SVD with Mitroflow prosthesis in aortic position in two patients above the age of 65 at 2 and 4 years after the first implantation. This is not the first report that questioned the durability of Mitroflow aortic valve (6-14). The overall incidence of SVD has been reported 13% (5). Alvarez et al. (7) describes their series of 491 patients >70 years of age received a Mitroflow aortic bioprosthesis. Freedom from SVD is 95% at 5 years but drops sharply to 55.8%in 10 years. According this author, the median time from operation to SVD is 48 months. Butany et al. (8), in a series of 12 bioprosthesis, observe SVD in 58% (n=7), and some degree of SVD in all valves implanted for over 2 years, which suggests that the Mitroflow pericardial valve is likely prone to early dysfunction, later requiring explantation. In fact, SVD consists of progressive stenosis with an unexpected and unpredictable life-threatening accelerated pattern in one third of SVD patients. Hence, early SVD in the Mitroflow bioprostheses necessitates annual echocardiography from the first year after the implantation in all patients and an even closer follow-up once the mean gradient reaches 30 mmHg. Owing to the life-threatening accelerated pattern of SVD in one third of patients, urgent reoperation should be considered once bioprostheses stenosis is severe, even in asymptomatic patients. In order to help the scientific cardiac surgery community better understand this problem and find a solution, we sought to add some histological evidences of early SVD in Mitroflow prosthesis. According to our observation the early SVD is related to a structural degeneration of the valve. Our microscopic examination reveals early collagen separation and fluid exudation seen in the valvular tissue likely contributing to the further damage. Our results suggest that the cusp degeneration are independent of calcification, since limited areas of calcification are observed in degenerated areas in one case. This means that SVD occurs as result of mechanical forces. Mitroflow dysfunction may be at least in part related to its design, likely to the cross stitches at the stent posts and the single piece of pericardium being wrapped around the outside of the Dacron-covered prosthesis stent. Perhaps the mechanical pressure, exerted on the para-stent post region of the cusps during the cardiac cycle, is enough to cause in some cases the tissue damage. To limit this damage related to mechanical forces, we think that the type and efficacy of prosthesis fixation needs to be optimized. Acellularization is a promising tool in the prevention of porcine aortic wall degeneration because reduces inflammatory reaction and collagen fragmentation. On the other hand, should be carefully evaluated the time interval from pericardial removal and starting of manufacturing to better treated animal tissue in order to reduce the immunogenicity that could also contribute to the triggering factors in the onset of tissue degeneration (15-17). Finally, despite the different risk factors previously described (12,13), our findings should warn surgeons and cardiologist as early SVD was not only found in the 19 but also in the 25-mm prosthesis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Brown JM, O’Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82-90. [Crossref] [PubMed]
- Yacoub MH, Takkenberg JJ. Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med 2005;2:60-1. [Crossref] [PubMed]
- Forcillo J, Pellerin M, Perrault LP, et al. Carpentier-Edwards pericardial valve in the aortic position: 25-years experience. Ann Thorac Surg 2013;96:486-93. [Crossref] [PubMed]
- Pomar JL, Jamieson WR, Pelletier LC, et al. Mitroflow pericardial bioprosthesis experience in aortic valve replacement > or =60 years of age. Ann Thorac Surg 1998;66:S53-6. [Crossref] [PubMed]
- Falk V, Baumgartner H, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2017;52:616-64. [Crossref] [PubMed]
- Bassano C, Gislao V, Bovio E, et al. An Unexpected Risk Factor for Early Structural Deterioration of Biological Aortic Valve Prostheses. Ann Thorac Surg 2018;105:521-7. [Crossref] [PubMed]
- Alvarez JR, Sierra J, Vega M, et al. Early calcification of aortic Mitroflo pericardial biprosthesis in the elderly. Interact Cardiovasc Thorac Surg 2009;9:842-6. [Crossref] [PubMed]
- Butany J, Feng T, Luk A, et al. Modes of failure in explanted mitroflow pericardial valves. Ann Thorac Surg 2011;92:1621-7. [Crossref] [PubMed]
- Asch FM, Heimansohn D, Doyle D, et al. Mitroflow aortic bioprosthesis 5-year follow-up: North American prospective multicenter study. Ann Thorac Surg 2012;94:1198-203. [Crossref] [PubMed]
- Roselli EE, Smedira NG, Blackstone EH. Failure modes of the Carpentier-Edwards pericardial bioprosthesis in the aortic position. J Heart Valve Dis 2006;15:421-7; discussion 427-8. [PubMed]
- Joshi V, Prosser K, Richens D. Early prosthetic valve degeneration with Mitroflow aortic valves: determination of incidence and risk factors. Interact Cardiovasc Thorac Surg 2014;19:36-40. [Crossref] [PubMed]
- Piccardo A, Blossier JD, Le Guyader A, et al. Fate of aortic bioprostheses: An 18-year experience. J Thorac Cardiovasc Surg 2016;151:754-61. [Crossref] [PubMed]
- Saleeb SF, Newburger JW, Geva T, et al. Accelerated degeneration of a bovine pericardial bioprosthetic aortic valve in children and young adults. Circulation 2014;130:51-60. [Crossref] [PubMed]
- Sénage T, Le Tourneau T, Foucher Y, et al. Early structural valve deterioration of Mitroflow aortic bioprosthesis: mode, incidence, and impact on outcome in a large cohort of patients. Circulation 2014;130:2012-20. [Crossref] [PubMed]
- Mosquera VX, Bouzas-Mosquera A, Velasco-García C, et al. Long-term outcomes and durability of the Mitroflow aortic bioprosthesis. J Card Surg 2016;31:264-73. [Crossref] [PubMed]
- Meuris B, Verbeken E, Flameng W. Prevention of porcine aortic wall calcification by acellularization: necessity for a non-glutaraldehyde-based fixation treatment. J Heart Valve Dis 2005;14:358-63. [PubMed]
- Manji RA, Zhu LF, Nijjar NK, et al. Glutaraldehyde fixed bioprosthetic heart valve conduits calcify and fail from xenograft rejection. Circulation 2006;114:318-27. [Crossref] [PubMed]


