Is sternal rewiring mandatory in surgical treatment of deep sternal wound infections?
Introduction
Deep sternal wound infections (DSWIs) are a rare but serious complication after median sternotomy and present a challenge for cardiac surgeons. In a single-center experience with 10,992 patients, Litwinowicz et al. described that 4% of all readmission to the intensive care unit is due to DSWI (1). While infection prevention should be prioritized in the face of DSWI, early and effective patient-tailored surgical treatment can be life-saving. Despite the urgent need, there are no consensus guidelines for the treatment of DSWIs. Cardiac surgeons typically treat DSWIs with sternal debridement and mediastinal irrigation, followed by drainage and sternal rewiring (2,3). Extensive sternal debridement with sternal plating in selected cases and sternectomy with subsequent muscle or omental flap plasty are often performed in patients treated by plastic surgeons (4-6). Negative pressure wound therapy (NPWT) is a simple and effective modality, that is used by both cardiac and plastic surgeons to improve peristernal blood flow and induce the development of granulation tissue (7,8). Litwinowicz et al. reported the effectiveness of hyperbaric oxygen therapy after failure of conventional treatment in 8 out of 10 patients, who developed DSWI (9). Removal of infected sternal wires seems to be the essential step in infection control. After extensive sternal debridement, severe fragmentation of the sternum may render the sternum unsalvageable. However, no European, American or other guideline is available to guide therapeutic decision-making in the treatment of various types of DSWIs. Further, no randomized controlled multicenter trials have reported the effects of different therapeutic modalities for this potentially life-threatening complication.
Over the past decade, new methods have been introduced that reportedly reduce the incidence of deep and superficial sternal wound, including platelet- rich plasma, Gentamicin- coated sponges, and incisional NPWT (10-12). Primary sternal plating is also reported to decrease the incidence of sternal wounds significantly (13-15). However, after radical sternal debridement of a DSWI is performed, sternal plating can be used only in selected cases, i.e., where there is no excessive bone loss or osteoporosis. There have been several previous reports about treating this complication of cardiac surgery with plastic surgery methods (16-18). Here, we compared the outcomes after surgical reconstruction with conventional sternal rewiring to those following interventions without sternal rewiring for the treatment of DSWIs.
Methods
Study design
We retrospectively evaluated all patients who underwent an open-heart procedure with a median sternotomy at the Department of Cardiac Surgery, at the St. Rafael County Hospital, Zalaegerszeg, Hungary, between January 2012 and December 2016. Patients diagnosed with a DSWI (according to the Centers for Disease Control and Prevention criteria) (19), were enrolled for analysis. Patients who did not receive surgical treatment were excluded. Prophylactic perioperative antibiotic therapy was standard throughout the study period. In each case, after the diagnosis of DSWI was established, the wound is explored and three tissue samples were taken for microbiological evaluation. Broad- spectrum antibiotic therapy was initiated and modified later according to the microbiological results. Based on the surgeon’s decision sternal rewiring (sternal rewiring group) or other intervention with no sternal rewiring (no sternal rewiring group) was performed. Other interventions included sternal debridement without rewiring, hemi- or total sternal resection, and bilateral pectoral muscle flap plasty in combination with Redon drainage.
The study was conducted in accordance with the Declaration of Helsinki. The institutional ethics committee approved the study (approval No. 10/2017).
Definitions
Sternal rewiring group: in this group, the sternum was rewired after debridement. Rewiring was performed using stainless steel wires, which were passed parasternally in a horizontal or horizontal and longitudinal manner, depending on the surgeon’s assessment.
No sternal rewiring group: in this group, no sternal wires were used in the final reconstruction. The sternum was either resected or left mechanically unfixed (in chronic cases) until subsequent bilateral pectoral muscle flap advancement plasty was performed.
In this report, the term “primary sternal wiring” refers to the primary cardiac operation closure.
End-points and data collection
The primary end-points were the need for readmission or death within 90 days. Hospital length of stay was compared between the two groups. The follow-up period was 12 months. Clinical and survival data were collected from the hospital medical records (Medworks Premium, St. Rafael County Hospital, Zalaegerszeg, Hungary) and analyzed retrospectively.
Surgical procedures
Figure 1 summarizes the various surgical procedures performed in the two groups. During the surgical exploration following the diagnosis of DSWI, we evaluated sternal fractures, necrosis, and wire tearing to determine sternal viability. In the sternal rewiring group, the sternal wires were removed, wound debridement was performed, and NPWT was administered (Vivano System, Hartmann Ltd., Budapest, Hungary). Antibiotic therapy was initiated and continued for four weeks after the surgical reconstruction. If the microbiological cultures were negative, the sternum was rewired according to the surgeon’s preference. In this group, final surgical reconstruction was performed in every case only after all microbiological cultures returned negative.
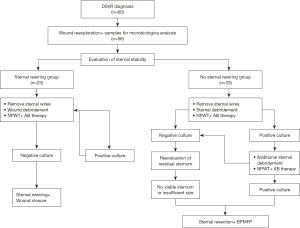
In the no sternal rewiring group, after removal of the sternal wires, thorough sternal debridement was performed. In the setting of negative wound cultures, the residual sternum was reevaluated for viability and size. Where non-viability or insufficient sternum size was identified, the sternum was wholly or partially resected based on the intraoperative findings and the discretion of the surgeon. Bilateral pectoral muscle flap plasty was performed over two Redon drains. When bacterial cultures remained positive, total sternectomy was performed, as the sternal osteomyelitis was considered the focus of infection, and the wounds were covered with a bilateral pectoral muscle flap plasty (Figure 2).
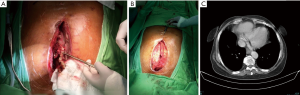
In cases of early surgical reconstruction (within four weeks after the primary sternal wiring), radical sternal debridement was performed after removing all sternal wires, and NPWT was administered for 2 weeks with dressing changes every 4 days. The use of NPWT usually stimulated the development of granulation tissue and stabilization of the sternal halves. Subsequently, the sternal defect or the defect between the sternal halves was covered by overlapping bilateral pectoralis muscle advancement flaps. In late and chronic cases of surgical reconstruction (more than four weeks after the primary sternal wiring), the wounds were explored, and sternal debridement was performed after all wires were removed. Then, NPWT was applied as described above. In most cases, the sternal halves were fixed in an open position by granulation tissue, and we did not attempt to re-fix the sternum with wires. Instead, we covered the sternum and the defect between the sternal halves with overlapping bilateral pectoralis muscle flaps (Figure 3).
Statistical analysis
We used IBM SPSS Statistics, Version 20 (IBM Corporation, Armonk, NY, US) for all statistical analyses. Categorical variables were compared between groups using the chi-squared test and are presented as numbers or percentages. Continuous variables were analyzed using the Mann-Whitney test and are presented as mean ± standard deviation. The Mantel-Haenszel chi-square test was applied to measure linear association in a cross tabulation. P<0.05 was considered statistically significant.
Results
During the study period, 3,177 patients underwent open heart surgery with sternotomy, and a DSWI was diagnosed in 60 patients (1.9%). Four patients were excluded because they died of overwhelming sepsis before any surgical reconstruction could be undertaken. In the remaining 56 cases, sternal rewiring was performed in 23 patients (41%), and intervention with no sternal rewiring was performed in 33 (59%) (Table 1).

Full table
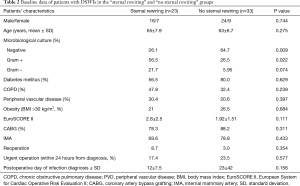
Table 2 summarizes the baseline data by patient group.
Full table
No significant between-group differences in sex, age, risk factors, or infection timing were observed. However, microbiological cultures were significantly different between the two groups. More gram-positive bacteria, mainly Staphylococcus aureus, were cultured from wounds in the sternal rewiring group than from wounds in the no sternal rewiring group (56.5% vs. 26.5%, respectively; P=0.022). The distribution of gram- negative wound cultures in the two groups showed no significant difference (21.7% vs. 5.95%, respectively; P=0.074, Table 2).
The rate of readmission was higher in the sternal rewiring group than in the no sternal rewiring group (63.6% vs. 14.7%, respectively; P<0.001). Readmissions were usually due to early excessive wound discharge, symptomatic sternal instability, or chronic fistula. The overall 90-day mortality rate was 8.9% [5/56; sternal rewiring, 21.7% (5/23) vs. no sternal rewiring, 0%, (0/33); P=0.030]. No additional deaths occurred during the 12-month follow-up period. Further, the median length of hospitalization was significantly longer in the sternal rewiring group than in the no sternal rewiring group (51 vs. 30 days; P=0.006).
Discussion
We compared the treatment outcomes after conventional sternal rewiring and reconstruction without sternal rewiring in patients with DSWIs and found higher readmission and early mortality rate in the sternal rewiring group. Conventional sternal rewiring is still the preferable procedure in treating DSWIs by cardiac surgeons. Reconstruction with muscle flaps is typically performed by plastic surgeons, and a significant variability was reported between the early mortality rates of plastic surgeons. Landes et al. reported a 30-day mortality of 12.5% after plastic surgical reconstruction of DSWIs (16). Cabbabe et al. reported more than four times mortality rate in patients treated first by cardiovascular surgeons using conventional methods than in those treated immediately by plastic surgeons, (4.7% vs. 1%) (17). We believe that advancement pectoral muscle flap plasty can be a first-line surgical approach to treat sternal wound infections as well as to manage the symptomatic unstable sternum (20). Pectoral muscle flap plasty can be easily performed by cardiac surgeons.
Stable sternal osteosynthesis, appropriate tissue perfusion, and sterile environment are the three main elements necessary for uncompromised sternal wound healing. All of these factors must be kept in mind during sternal wound reconstruction. The surgical reconstruction of sternal wounds performed by cardiac surgeons is characterized by attempts to preserve the sternum and develop a biomechanically stable osteosynthesis. These procedures may involve a somewhat conservative sternal debridement and subsequent insertion of more wires into the infected wound (17). Where tissue perfusion is inadequate and in cases of poor microcirculation, (e.g., bilateral internal thoracic artery harvesting, obesity, diabetes mellitus, and peripheral vasculopathy), a stable osteosynthesis may not be sufficient for effective wound healing. Wound sterility is difficult to assess before surgical reconstruction. The first tissue samples may be more informative if obtained before antibiotic therapy is initiated. However, subsequent specimens are usually acquired after the administration of antibiotics and may provide false-negative results. Our study results suggest, that adherence to sternal preservation using rewiring to build a new osteosynthesis without considering the significance of tissue perfusion may lead to infection control failure and, consequently, failure of the surgical reconstruction. In this study, we report our results in patients who had enough granulation tissue to fix the sternal halves without any wires left in the wounds. In these cases, subsequent reconstruction was achieved using bilateral pectoral muscle advancement flaps.
The role of positive wound cultures at the time of reconstruction remains controversial. Rodriguez et al. reported that negative microbiological cultures were not mandatory before surgical wound closure (21). In our experience, some culture-positive DSWIs can be reconstructed with good results at the expense of radical surgical debridement. However, this is not our usual approach to wound care. Here, we found that positive wound cultures contributed significantly to treatment failure, especially when sternal refixation was attempted by conventional wiring. The role of foreign bodies, (i.e., wires and plates), in sternal wound infection recurrence has not been thoroughly examined. Elgharably et al. showed biofilms on sternal wires removed from the sternal wounds of six patients (22). Their findings may indicate reflect the importance of eliminating all foreign bodies from infected wounds to obtain a sterile wound environment. Radical surgical debridement, even at the expense of partial or total sternectomy, followed by the application of plastic surgical techniques seems to be a more effective approach to sternal wound treatment than the use of conventional cardiac surgical methods. We prefer to prepare bilateral unpedicled rotational advancement flaps and unite them in an overlapping manner at the wound midline instead of using turnover flaps in patients who have undergone internal mammary artery harvesting.
Limitations
This is a single-center retrospective study; the sample size is small, and the follow-up period is short. Therefore, no further conclusions can be drawn.
Gram-positive bacterial cultures occurred more frequently in the sternal rewiring group than in the no sternal rewiring group. However, final sternal rewiring was only performed after microbiological cultures became negative.
Conclusions
This study indicates that reconstructing the infected sternum with additional wires may not be the most appropriate strategy in many patients with DSWIs. Extensive sternal debridement and elimination of all potential nidi of infection, followed by wound closure with well-vascularized muscle flaps, may lead to a decreased rate of surgical treatment failure.
Acknowledgements
None.
Footnote
Conflicts of Interest: This paper was partially presented at the 31st Annual Meeting of The European Association of Cardiothoracic Surgery, Vienna, Austria, October 7-11, 2017.
Ethical Statement: The study was conducted in accordance with the Declaration of Helsinki. The institutional ethics committee approved the study, (Approval No. 10/2017).
References
- Litwinowicz R, Bartus K, Drwila R, et al. In-hospital mortality in cardiac surgery patients after readmission to the intensive care unit: a single-center experience with 10,992 patients. J Cardiothorac Vasc Anesth 2015;29:570-5. [Crossref] [PubMed]
- Bryant LR, Spencer FC, Trinkle JK. Treatment of median sternotomy infection by mediastinal irrigation with an antibiotic solution. Ann Surg 1969;169:914-20. [Crossref] [PubMed]
- Shumacker HB Jr, Mandelbaum I. Continuous antibiotic irrigation in the treatment of infection. Arch Surg 1963;86:384-7. [Crossref] [PubMed]
- Singh K, Anderson E, Harper JG. Overview and management of sternal wound infection. Semin Plast Surg 2011;25:25-33. [Crossref] [PubMed]
- Juhl AA, Koudahl V, Damsgaard TE. Deep sternal wound infection after open heart surgery--reconstructive options. Scand Cardiovasc J 2012;46:254-61. [Crossref] [PubMed]
- Parissis H, Al-Alao B, Soo A, et al. Risk analysis and outcome of mediastinal wound and deep mediastinal wound infections with specific emphasis to omental transposition. J Cardiothorac Surg 2011;6:111. [Crossref] [PubMed]
- Morisaki A, Hosono M, Murakami T, et al. Effect of negative pressure wound therapy followed by tissue flaps for deep sternal wound infection after cardiovascular surgery: propensity score matching analysis. Interact Cardiovasc Thorac Surg 2016;23:397-402. [Crossref] [PubMed]
- Yu AW, Rippel RA, Smock E, et al. In patients with post-sternotomy mediastinitis is vacuum-assisted closure superior to conventional therapy? Interact Cardiovasc Thorac Surg 2013;17:861-5. [Crossref] [PubMed]
- Litwinowicz R, Bryndza M, Chrapusta A, et al. Hyperbaric oxygen therapy as additional treatment in deep sternal wound infections - a single center's experience. Kardiochir Torakochirurgia Pol 2016;13:198-202. [Crossref] [PubMed]
- Patel AN, Selzman CH, Kumpati GS, et al. Evaluation of autologous platelet rich plasma for cardiac surgery: outcome analysis of 2000 patients. J Cardiothorac Surg 2016;11:62. [Crossref] [PubMed]
- Schimmer C, Ozkur M, Sinha B, et al. Gentamicin-collagen sponge reduces sternal wound complications after heart surgery: a controlled, prospectively randomized, double-blind study. J Thorac Cardiovasc Surg 2012;143:194-200. [Crossref] [PubMed]
- Hunter JE, Teot L, Horch R, et al. Evidence-based medicine: vacuum-assisted closure in wound care management. Int Wound J 2007;4:256-69. [Crossref] [PubMed]
- Gottlieb LJ, Pielet RW, Karp RB, et al. Rigid internal fixation of the sternum in postoperative mediastinitis. Arch Surg 1994;129:489-93. [Crossref] [PubMed]
- Lee JC, Raman J, Song DH. Primary sternal closure with titanium plate fixation: plastic surgery effecting a paradigm shift. Plast Reconstr Surg 2010;125:1720-4. [Crossref] [PubMed]
- Song DH, Lohman RF, Renucci JD, et al. Primary sternal plating in high-risk patients prevents mediastinitis. Eur J Cardiothorac Surg 2004;26:367-72. [Crossref] [PubMed]
- Landes G, Harris PG, Sampalis JS, et al. Outcomes in the management of sternal dehiscence by plastic surgery: a ten-year review in one university center. Ann Plast Surg 2007;59:659-66. [Crossref] [PubMed]
- Cabbabe EB, Cabbabe SW. Immediate versus delayed one-stage sternal debridement and pectoralis muscle flap reconstruction of deep sternal wound infections. Plast Reconstr Surg 2009;123:1490-4. [Crossref] [PubMed]
- Brandt C, Alvarez JM. First-line treatment of deep sternal infection by a plastic surgical approach: superior results compared with conventional cardiac surgical orthodoxy. Plast Reconstr Surg 2002;109:2231-7. [Crossref] [PubMed]
- Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988;16:128-40. [Crossref] [PubMed]
- Cabbabe EB, Cabbabe SW. Surgical management of the symptomatic unstable sternum with pectoralis major muscle flaps. Plast Reconstr Surg 2009;123:1495-8. [Crossref] [PubMed]
- Rodriguez Cetina Biefer H, Sundermann SH, Emmert MY, et al. Negative microbiological results are not mandatory in deep sternal wound infections before wound closure. Eur J Cardiothorac Surg 2012;42:306-10. [Crossref] [PubMed]
- Elgharably H, Mann E, Awad H, et al. First evidence of sternal wound biofilm following cardiac surgery. PLoS One 2013;8:e70360. [Crossref] [PubMed]


